
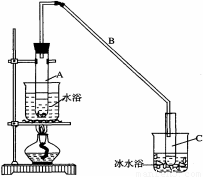
ij��ѧС���������װ�ã���ͼһ�����Ի������Ʊ�����ϩ����֪��
ͼһ[
(1)�Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ������B���˵�������е�������
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ��
(2)�Ʊ���Ʒ
ͼ��
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��________�㣨��ϡ����¡�������Һ����________�������ţ�ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ��(ͼ��)װ��������ȴˮ�� �ڽ���(����ĸ)������ʱҪ������ʯ�ң�Ŀ���� ��
![]() ���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� ���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���ǣ� ��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� ���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���ǣ� ��
A������ʱ��70�濪ʼ�ռ���Ʒ B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
A�������Ը��������Һ B���ý����� C���ⶨ�е�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| 16m3 |
| m-m3 |
| 16m3 |
| m-m3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ��̨�и���3����Ͽ��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
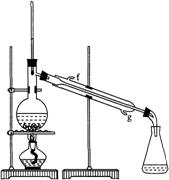
ij��ѧС�������ͼװ��ģ���ⱥ��ʳ��ˮ�Ʊ�������ͨ��������ԭ����ͭ�ⶨCu�����ԭ��������ͬʱ���������������ԣ�ͼ�мгֺͼ��������Ѿ���ȥ����

��1��д��װ�ü��з�Ӧ�����ӷ���ʽ ��Ϊ�������ʵ�飬��ȷ��������ʽΪa���� ��b���� ������ĸ����
��2����װ�����ձ���Һ��������� ��Aƿ��ʢװ����Һ����� ������ĸ����
a��I-������Һ b��NaOH��Һ
c��FeCl2��KSCN�����Һ d��Na2SO3��Һ
�ڼ���װ�ñ��е�����ͭ��ĩ֮ǰ������Ҫ����װ�õ������Ի�����еı�Ҫ������ ��
��3������װ�ñ��ⶨCu�����ԭ���������������ַ������ٲ�÷�Ӧǰ��ϴ��ƿB������Һ��������m1���ڲ�÷�Ӧǰ��U�ιܼ����й���������m2������Ϊ�����ķ���Ϊ ����١��ڡ����������ò�÷�Ӧ��Ӳ�ʲ�������ʣ����������m3�ķ�������֪O�����ԭ������Ϊ16��ʵ��������ͭ��Ʒ����Ϊm����ⶨCu�����ԭ�������ı���ʽΪ ���÷����ڷ�Ӧ��Ӳ�ʲ�������ȴ������û��һֱͨ�������ᵼ�²ⶨCu�����ԭ������ ���ƫ����ƫС������Ӱ�족���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ɶ���2009-2010ѧ��߶�4���¿���ѧ���� ���ͣ������
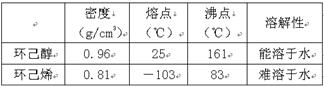
ij��ѧС���������װ�ã���ͼһ�����Ի������Ʊ�����ϩ����֪��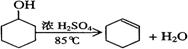
ͼһ[
(1)�Ʊ���Ʒ
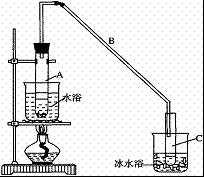
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ������B���˵�������е�������
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ��
(2)�Ʊ���Ʒ
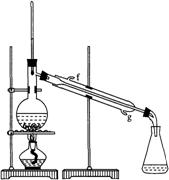
ͼ��
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��________�㣨��ϡ����¡�������Һ����________�������ţ�ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ��(ͼ��)װ��������ȴˮ�� �ڽ���(����ĸ)������ʱҪ������ʯ�ң�Ŀ���� ��
 ���ռ���Ʒʱ�����Ƶ��¶�Ӧ��
���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���ǣ� ��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��
���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���ǣ� ��
A������ʱ��70�濪ʼ�ռ���Ʒ B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
A�������Ը��������Һ B���ý����� C���ⶨ�е�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��) ij��ѧС���������װ�ã���ͼһ�����Ի������Ʊ�����ϩ����֪��![]()
ͼһ[
(1)�Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ![]() ������B���˵�������е�������
������B���˵�������е������� ![]()
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ![]() ��
��
(2)�Ʊ���Ʒ
ͼ��
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��________�㣨��ϡ����¡�������Һ����________�������ţ�ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ��(ͼ��)װ��������ȴˮ�� �ڽ���(����ĸ)������ʱҪ������ʯ�ң�Ŀ���� ��
| |
A������ʱ��70�濪ʼ�ռ���Ʒ B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
A�������Ը��������Һ B���ý����� ![]() C���ⶨ�е�
C���ⶨ�е�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com