
���� ��1���ٲ���ֱ�ӻ�ϵ�ԭ����Fe2+�ڼ��������¸����ױ�������
�ڸ����������Ϣ�������ķ�ӦΪ��NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O��
�����Ŀ����е�O2������Fe2+����ֹFe2+��������
��2����������ΪLiCoO2������ӵ�أ�����������������Ҫ����LiCoO2������AI��Fe�ȣ�������ϡ�����ܽ���������ܽ�����������������������������������ƣ�S2O32-��������SO42-�����ɵ���Һ�к�������ﮡ������ܡ������ƣ���������������Һ��ͨ�����������������Ϊ�����ӣ��γ������ӡ������ӵij�������Һ�м��������������Ƶ�����ҺPH�������ܣ����˵õ��������ܣ���Һ�м����������ơ�̼���Ƶ�����ҺPH����������γ�̼��ﮣ�
��ͨ�������Ϣ��֪LiCoO2��Na2S2O3������������ԭ��Ӧ����ӦΪ8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O��
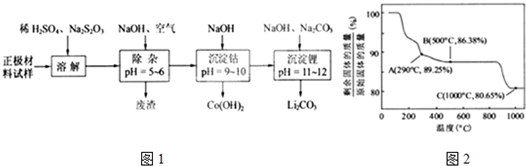
�ڸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬ͨ��������ݿ�����1000����Co��OH��2��ȫ�ֽ⣬�����CoO��
��350-400��ʱ�������������89.25%-86.38%֮�䣬����ͨ��������з�����
��290�棬n��Co����n��O��=$\frac{100}{93}$��[��89.25-100��$\frac{59}{93}$����16]=2��3���仯ѧʽΪCo2O3��
��500�棬n��Co����n��O��=$\frac{100}{93}$��[��86.38-100��$\frac{59}{93}$����16]=3��4���仯ѧʽΪCo3O4��
���Կ���ȷ����350-400��ʱ�Ļ�ѧʽΪCo2O3��Co3O4��
��� �⣺��1����NH4��2Fe��SO4��2��LiOH��Һ��Ӧ����Fe��OH��2��Fe��OH��2�ױ��������������Բ��ܽ�ֱ�ӻ�ϣ�
�ʴ�Ϊ��Fe2+�ڼ��������¸��ױ�������
�ڸ��������Ϣ����NH4��2��H3PO4��LiOH��Һ������������Ӧ����LiFePO4��NH4HSO4��H2O����Ӧ�Ļ�ѧ����ʽΪ����NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4+2NH4HSO4+H2O��
�ʴ�Ϊ����NH4��2Fe��SO4��2+LiOH+H3PO4=LiFePO4��+2NH4HSO4+H2O��
�۸��³���ǰ������LiFePO4�м�����������̿�ڣ������ó��˿��Ը��Ƴ��ͺ��LiFePO4�ĵ��������⣬�������Ŀ����е�����������Fe2+����ֹFe2+��������
�ʴ�Ϊ���������O2��Ӧ����ֹLiFePO4�е�Fe2+��������
��2����������������Ҫ����LiCoO2������Al��Fe�ȣ�����ϡH2SO4��Na2S2O3��S2O32-��������SO42-�����л�ԭ�ԣ�����������ֻ��LiCoO2���������ԣ��뷴ӦNa2S2O3��Ӧ����CoSO4����Ӧ��ѧ����ʽΪ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O��
�ʴ�Ϊ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O��
�ڸ��������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬����ԭʼ��������Ϊ100g����n��Co��=$\frac{100}{93}$mol��m��Co��=100��$\frac{59}{93}$g��
��1000��ʱ�������������ٱ仯��˵��Co��OH��2��ȫ�ֽ⣬n��Co����n��O��=$\frac{100}{93}$��[��80.65-100��$\frac{59}{93}$����16]=1��1��ʣ�����ɷ�ΪCoO��
��350-400��ʱ�������������89.25%-86.38%֮�䣬����ͨ��������з�����
��290�棬n��Co����n��O��=$\frac{100}{93}$��[��89.25-100��$\frac{59}{93}$����16]=2��3���仯ѧʽΪCo2O3��
��500��n��Co����n��O��=$\frac{100}{93}$��[��86.38-100��$\frac{59}{93}$����16]=3��4���仯ѧʽΪCo3O4��
���Կ���ȷ����350-400��ʱ�Ļ�ѧʽΪCo2O3��Co3O4��
�ʴ�Ϊ��CoO��Co2O3��Co3O4��
���� ���⿼���֪ʶ�Ƚ�ɢ���漰����Դ���ã��������ʡ��������̷�����ͼ��������������ȽϹ㣬��Ŀ�ѶȽϴ�


 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ʒ�Ӧ��2CH3CH2OH+2Na��2CH3CH2ONa+H2�� | |
| B�� | ��������ȼ�շ�Ӧ��2CH3CH2OH+O2$\stackrel{��ȼ}{��}$2CH3CHO+2H2O | |
| C�� | �������Ĵ�������CH3CH2OH+3O2$��_{��}^{Cu��Ag}$2CO2+3H2O | |
| D�� | �������������Ӧ��CH3CH2OH+CH3COOH$��_{��}^{����}$CH3CH2OCH2CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
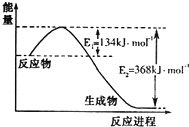 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���ش�
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���ش�| t/K | 298 | 398 | 498 | �� |
| K/��mol•L-1�� | 4.1��106 | K1 | K2 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ȥ�����е�HCl���� | |
| B�� |  ����Na2CO3��Һ��CH3COOC2H5�Ļ���� | |
| C�� |  ������ȡ��������ã��²���ҺΪ�Ⱥ�ɫ���ϲ�Ϊ��ɫ | |
| D�� | 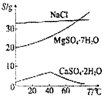 �ܽ�ȱ仯��֪���ڽϵ��¶���������MgSO4•7H2O��CaSO4•2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
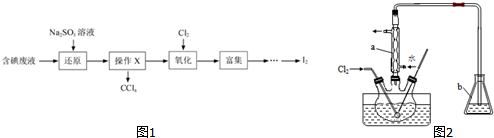
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ��Ŀ�� | ʵ����� | ʵ��ԭ�� | |
| A | ��֤Fe��OH��3���ܽ��С��Mg��OH��2 | ��FeCl3��Һ����Mg��OH��2����Һ�У��� | 3Mg��OH��2+2Fe3+?2Fe��OH��3+3Mg2+ |
| B | ����FeCl3��Һ | ��FeCl3�����ܽ�������������Һ | H+����FeCl3ˮ�� |
| C | ������Һ���Ƿ���NH4+ | ȡ������Һ���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������� | NH3����ˮ�����ʵ���Ҫ������ʽ��NH4+��OH- |
| D | �ᴿ������������ص��Ȼ��� | �ڽϸ��¶����Ƶ�Ũ��Һ����ȴ�ᾧ�����ˡ����� | �Ȼ����ܽ�����¶����߱仯����������ܽ�����¶������������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
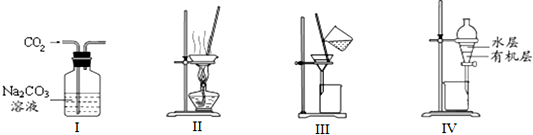
| A�� | ��ͼI��ȥCO2�к��е�����Cl2 | |
| B�� | ��ͼ������NH4Cl������Һ�Ʊ�NH4Cl���� | |
| C�� | ��ͼ�����NaCl��Na2SO4�Ļ����Һ | |
| D�� | ��ͼ������CCl4��ȡ��ˮ�еĵ���ѷֲ���л����ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ȵμ���ˮ���ٵμ�KSCN��Һ���Ժ�ɫ | |
| B�� | �ȵμ�KSCN��Һ�����Ժ�ɫ���ٵμ���ˮ���Ժ�ɫ | |
| C�� | �μ�NaOH��Һ����������ɫ���������ʺ��ɫ | |
| D�� | ֻ��μ�KSCN��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ��Ӧ�����Ǻ�����ѧ��Ӧ���п����̶ȵ������� | |
| B�� | ��ѧ��Ӧ����ͨ���õ�λʱ�������ɻ�����ij���ʵ������Ķ�������ʾ | |
| C�� | ��һ�����淴Ӧ�ﵽƽ��״̬ʱ������������Ӧ���ܴﵽ���ȣ���ʹ�����ı䣬���ȶ�����ı� | |
| D�� | ƽ��״̬��һ�־�ֹ��״̬����Ϊ��Ӧ����������Ũ���Ѿ����ٸı� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com