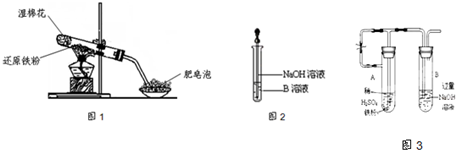
ijͬѧ������ͼ1��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ�飬�������о������仯����IJ������ʣ�
��֪����FeO+2H
+=Fe
2++H
2O��Fe
2O
3+6H
+=2Fe
3++3H
2O ��Fe
3O
4+8H
+=Fe
2++2Fe
3++4H
2O
��ش��������⣺
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ
��
��2����ͬѧ��ȷ����Ӧһ��ʱ���Ӳ���Թ��й������ʵijɷ֣����������ʵ�鷽����
�ٴ�Ӳ���Թ���ȴ��ȡ�������еĹ�����������ϡ�������ҺB��
��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��ǣ�ֻ��һ��ѡ��������⣩
A
A
������Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��ǣ�ֻ��һ��ѡ��������⣩
C
C
��
A��һ����Fe
3O
4��������Fe B��ֻ��Fe��OH��
3C��һ����Fe
3O
4��Fe
D��һ����Fe��OH��
3��������FeE��ֻ��Fe
3O
4��3����ͬѧ������ʵ�鷽��������ʵ�飬�����Һδ���ɫ��ԭ����
Fe+2Fe3+=3Fe2+
Fe+2Fe3+=3Fe2+
�������ӷ���ʽ��ʾ����
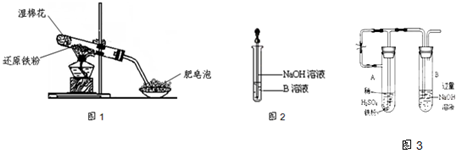
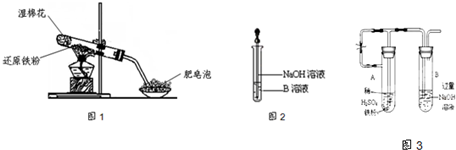
��4����ͬѧ������ȡ������ҺB��ʹ���NaOH��Һ��Ӧ������ͼ2��ʾ�IJ������ɹ۲쵽���ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��������д��������������صķ�Ӧ�Ļ�ѧ����ʽ
FeSO4+2NaOH=Fe��OH��2��+Na2SO4��4Fe��OH��2+O2+2H2O=4Fe��OH��3
FeSO4+2NaOH=Fe��OH��2��+Na2SO4��4Fe��OH��2+O2+2H2O=4Fe��OH��3
��
��5��һ��ʱ���ͬѧ���֣�3����δ������Һ��ɺ�ɫ��˵��Fe
2+����
��ԭ
��ԭ
�ԣ��ɴ˿�֪��ʵ�����к�Fe
2+������Һ�����������Ƶ�ԭ����
Fe2+�ױ������е���������������
Fe2+�ױ������е���������������
���������ƺ�Fe
2+������ҺʱӦ��������
����
����
��
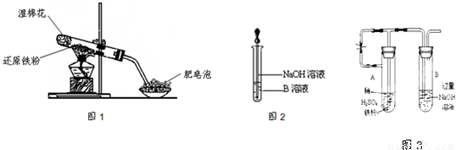
��6����ͬѧΪ�˻�ó־ð�ɫ��Fe��OH��
2����������ͼ3��ʾװ�ã��ò���O
2������ˮ���Ƶ�NaOH��Һ�����Ƶ�FeSO
4��Һ��Ӧ����ò���O
2������ˮ�ķ�����
������ˮ���
������ˮ���
����Ӧ��ʼʱ����ֹˮ�е�Ŀ����
�������ۺ�ϡ���ᷴӦ�����������ų��Թ�A��B�еĿ�������ֹFe��OH��2����������������
�������ۺ�ϡ���ᷴӦ�����������ų��Թ�A��B�еĿ�������ֹFe��OH��2����������������
��
һ��ʱ��ر�ֹˮ�У����Թ�
B
B
���A����B�����й۲쵽��ɫ��Fe��OH��
2��