
 ����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳ�����ˮ���ᣬ�Ʊ�����Ҫ��ӦΪ��
����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳ�����ˮ���ᣬ�Ʊ�����Ҫ��ӦΪ��

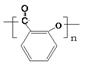 ��2�֣�
��2�֣�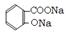 ��4����
��4����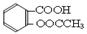 +3NaOH
+3NaOH CH3COONa+2H2O+ ��2�֣�
CH3COONa+2H2O+ ��2�֣� ����3���ֲ�Ʒ�ᴿ���� ��������������NaHCO3�ܽ�ֲ�Ʒ��Ŀ����ʹ����ˮ����ת��Ϊ������ˮ������ˮ�����ƣ�������ۺ�����롣�жϸù��̽����ķ�����û��CO2�������� ��Һ��������Ũ�����У��������������л��Dz������� �������ղ�Ʒ���Ƿ���ˮ����Ļ�ѧ������ȡ�����ᾧ���Թ��У�������ˮ�ܽ⣬�μ�FeCl3��Һ����������ɫ��ˮ���ᡣ��4���� ����ˮ���������NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽ
����3���ֲ�Ʒ�ᴿ���� ��������������NaHCO3�ܽ�ֲ�Ʒ��Ŀ����ʹ����ˮ����ת��Ϊ������ˮ������ˮ�����ƣ�������ۺ�����롣�жϸù��̽����ķ�����û��CO2�������� ��Һ��������Ũ�����У��������������л��Dz������� �������ղ�Ʒ���Ƿ���ˮ����Ļ�ѧ������ȡ�����ᾧ���Թ��У�������ˮ�ܽ⣬�μ�FeCl3��Һ����������ɫ��ˮ���ᡣ��4���� ����ˮ���������NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽ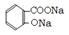
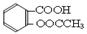 +3NaOH
+3NaOH CH3COONa+2H2O+ ���� ���ݷ�Ӧ����ʽ���ù�ϵʽ��
CH3COONa+2H2O+ ���� ���ݷ�Ӧ����ʽ���ù�ϵʽ��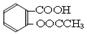 �D3NaOH�����ݵζ����������ǵõ�����ˮ����������
�D3NaOH�����ݵζ����������ǵõ�����ˮ����������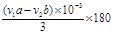 g����˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ
g����˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ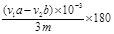 ��
��

 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C3H4 | B��CH4 | C��C3H8 | D��C2H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������������������NaOH��Һ����Һ |
| B�����飨��ϩ���������� |
| C���Ҵ���ˮ������������ʯ�ң����� |
| D����֬������Ӧ�����з������֬�����ƣ���NaCl������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ը������ | B����ˮ | C��Һ�� | D��������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


| | �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com