
��ͼ��A��B��C��D��EΪ���ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��C+G��B+H���ų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�G�����Ǵ��������Ҫ�ɷ֣���I��һ�ֳ������������壬����E������Ӧ��2E+I��2F+D��F��EԪ�ص���������Ϊ60%���ش��������⣨ÿ��2�֣���12�֣�
��1����Ӧ�ٵĻ�ѧ����ʽ��
��2��������I�������ڵĻ�ѧ���� ��������ӡ����ԡ��Ǽ��ԡ���
��3����ȡ11.9gB��C��E�Ļ����ù�����NaOH��Һ�ܽ���ˡ�����ʣ���������Ϊ9.2g����ȡ��������B��C��E�Ļ������ϡ������ȫ�ܽ⣬���ռ������������6.72L����ʣ��Ļ��Һ�����������NaOH��Һʹ���еĽ���������ȫ�����������������Ϊ�� ��
A��27.2g B��7.8g C��2.7g D��19.4g
(4)C�������NaOH��Һ��Ӧ�����ӷ���ʽ�ǣ�
��5����G���ڹ�����ϡ�����У����������е�Fe3+�ķ����� �����������е�Fe2+�ķ�����
A���μ�KSCN��Һ����Һ��Ѫ��ɫ
B�������ۣ���Һ��dz��ɫ
C����������KMnO4��Һ��Ѹ����ɫ
D���μ�NaOH��Һ���а�ɫ������Ѹ�ٱ�ɻ���ɫ���ת��Ϊ�� ��ɫ
��ɫ
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |


 ����1��
����1�� ����1��
����1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |




| ���� |

| ���� |
| ���� |

| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |



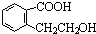
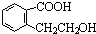
| ���� |
 CH2-CH2
CH2-CH2 n��CH2=CH2+H2O
n��CH2=CH2+H2O| ���� |
| ���� |
 CH2-CH2
CH2-CH2 n��CH2=CH2+H2O
n��CH2=CH2+H2O| ���� |
�鿴�𰸺ͽ���>>
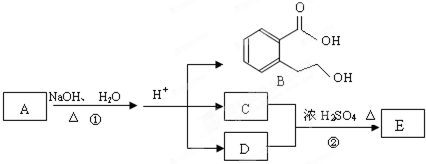
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����5�µ�һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
��ͼ��A��B��C��D��E��Ϊ�л��������֪��C����NaHCO3��Һ������Ӧ��C
��D����Է���������ȣ���D�������IJ��ﲻ�ܷ���������Ӧ��

�ش��������⣺
��1��C�����й����ŵ�������__________��������B���ܷ����ķ�Ӧ��_________��(����ĸ���) a���ӳɷ�Ӧb��ˮ�ⷴӦc����ȥ��Ӧd��������Ӧ
��2��д��E�Ľṹ��ʽ______________________________________��
��3��д����Ӧ�ٵĻ�ѧ����ʽ��_____________________________________________��
��4��ͬʱ������������������B��ͬ���칹���ж��֣�
a���������ж���ȡ�����ұ����ϵ�һ�ȴ�����2��
b������FeCl3��Һ������ɫ��Ӧ
c���ܷ���ˮ�ⷴӦ
��д��lmol B��ͬ���칹���У�����3molNaOH��Ӧ�Ľṹ��ʽ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��15�֣���ͼ��A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ�

��1����֪��AԪ�ص���ͼ�Ϊ-3�ۣ�������������ﺬ��56.34����ԭ�Ӻ�������������������1������AԪ��ԭ�ӵ�������Ϊ������ ����ԭ������Ϊ____ ___��AԪ����ԪԪ�����ڱ��е�λ��Ϊ___ __��
��2��д��Ԫ�ط���A__ ______B____ ____��C������ �� ����D_____ ____��
��3��A��B��C����Ԫ������������ˮ����Ļ�ѧʽΪ��������������������������������������������ǿ����_________��
��4��B��D����Ԫ�غ�����ɵ���̬�⻯��Ļ�ѧʽ����Ϊ__________������������������______ __�ȶ������____ ____��ԭ����ǿ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com