

| ������ | Fe��OH��3 | Al��OH��3 | Zn��OH��2 |
| ��ʼ����pH | 2.3 | 4.0 | 5.4 |
| ��ȫ����pH | 4.1 | 5.2 | 8.0 |




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
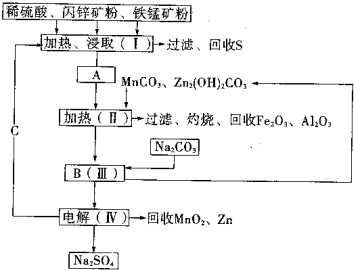 ��2011?����ģ�⣩ij���������̿�MnO2Լ70%������Al2O3������п��ZnSԼ80%������FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ������������£�
��2011?����ģ�⣩ij���������̿�MnO2Լ70%������Al2O3������п��ZnSԼ80%������FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ������������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� |
�鿴�𰸺ͽ���>>
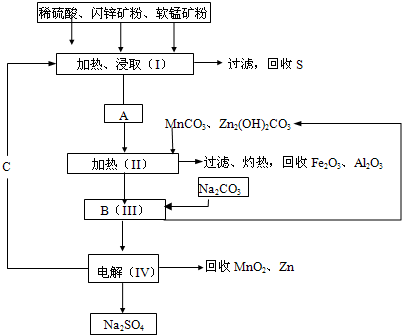
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ������ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ������
ij���������̿�MnO2Լ70%��Al2 O3������п��ZnSԼ80%��FeS����ͬ����MnO2����Zn���ɵ��ԭ�ϣ����������£�
��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O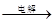 MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��
��1��A�����ڻ�ԭ������� ��
��2������MnCO3��Zn2(OH)2CO3�������� ��C�Ļ�ѧʽ�� ��
��3���������г��õ�Na2SO4��S�ȸ���Ʒ�⣬���ɵõ��ĸ���Ʒ�� ��
��4������ƷS�����������ᣬת�������ǣ�S��SO2��SO3��H2SO4��д���ڶ���ת���Ļ�ѧ����ʽ ��
��5��Ҫ��Na2SO4��Һ�еõ�â���� Na2SO4��10H2O��������еIJ���������Ũ���� ��
���ˡ�ϴ�ӡ�����ȡ�
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��Ͷ�ϵ������ȴ�Լ�� ��
�鿴�𰸺ͽ���>>
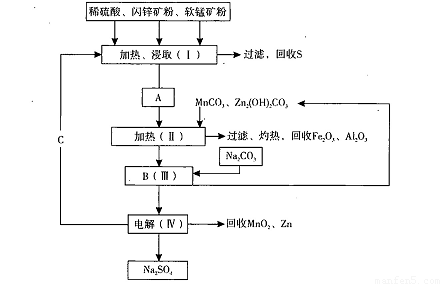
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�ع��и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
���̿��к�MnO2Լ70%��SiO2Լ20%��Al2O3Լ4%������Ϊˮ�֣���п���к�ZnSԼ80%��FeS��CuS��SiO2��Լ7%������Ϊˮ�֡�������Ա�������ۺ�������������Դ��ͬ��������գ���ȡZn��MnO2��Na2SO4���乤���������£�

��1��I����Һ�к���MnSO4��ZnSO4��CuSO4��Fe2(SO4)3��Al2(SO4)3�ȡ�д��MnO2��CuS�����ᷴӦ�Ļ�ѧ����ʽ�� ��
��2����֪Fe(OH)3��Al(OH)3��Zn(OH)2�������ʿ�ʼ��������ȫ����ʱ��Һ��pH���±���
|
������ |
Fe(OH)3 |
Al(OH)3 |
Zn(OH)2 |
|
��ʼ����pH |
2.3 |
4.0 |
5.6 |
|
��ȫ����pH |
4.1 |
5.2 |
8.0 |
��III�е�����Һ��pH��5.2��5.4����ʱ���ɳ���M�ijɷ�Ϊ ��д��ѧʽ����III�м���MnO2�������� ��
��3��Na2SO4��Na2SO4��10H2O���ܽ�����ߣ�g/100gˮ����ͼ����IV�еõ�Na2SO4����IJ����ǣ��������MnCO3��ZnCO3�����Һ���½ᾧ�� �����Ҵ�ϴ�Ӻ������Ҵ�ϴ�Ӷ�����ˮϴ��ԭ���� ��

��4��V���ö��Ե缫����Ƶ�Zn��MnO2���������ĵ缫��ӦʽΪ ��
��5����ɫ��ѧ˼���ڱ������еõ��˳�����֣��ڱ����������п�ѭ��ʹ�õ���Ҫ�����У�MnO2��ZnCO3��MnCO3�� �� ��д��ѧʽ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com