
����Ŀ���л����У���һЩ����ʽ����ͨʽCnHn����C2H2��C6H6�ȡ�
(1)������Щ�л����˵������ȷ����______(����)��
A�����ܻ�Ϊͬϵ�� |
B���ڿ�����ȼ��ʱ���������Ҳ������� |
C��һ����ʹ�������������Һ��ɫ |
D������������Щ�л�����ȫȼ��ʱ��������ͬ |
(2)д������ʽΪC4H4��Ϊ�������л���Ľṹ��ʽ__________������һ�ȴ����ͬ���칹����________�֡�
(3)д�����������ʽΪC6H6�ҽṹ��ֻ��C��C����C��H�����л���Ľṹ��ʽ��____________________________________________________________��
(4)ij�л������ʽΪC8H8�������ڷ���������֪����ʹ�������������Һ����ˮ��ɫ������л���Ľṹ��ʽΪ________��д���䷢���Ӿ۷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
���𰸡�(1)BD��
(2)HC��C��CH==CH2��3��
(3)��
(4)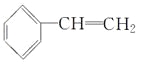 ��
�� ��
��
�������������������1��A��ͬϵ�����Ƚṹ���ƣ�C2H2����Ȳ����C6H6���ڷ�������������ͬϵ��ʴ���B��ͨʽΪCnHn����̼���ߣ��Һ�̼����ȣ����ȼ�չ����л��������Ҳ������̣�����ȷ��C��C6H6����DZ�������ʹ���Ը��������Һ��ɫ���ʴ���D��������ʱ�����ʽΪCH����˺�������ͬ������ȷ��(2)4��C��������������ΪC4H10����C4H4���6���⣬˵������3��̼̼˫����1��̼̼������1��̼̼˫����ͬһ��̼ԭ���ϲ�����������̼̼˫�����������л��ﲻ�ȶ�����˴��л���ΪHC��C��CH=CH2����3�ֲ�ͬ����ԭ�ӣ���һ�ȴ�����3�֣���3��ֻ��C��C����C��H����˵���DZ��͵ģ����������������ʽΪC6H14����˲����������ṹ��ʽֻ����![]() ����4�����ڷ����������б�������ʹ���������Һ����ˮ��ɫ�����в����ͼ�����˽ṹ��ʽΪ��
����4�����ڷ����������б�������ʹ���������Һ����ˮ��ɫ�����в����ͼ�����˽ṹ��ʽΪ�� ��
�� ��
��


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й�����Һ�ͽ������������ȷ����( )
A����Һ�ǵ����Եģ������Ǵ����
B��ͨ��ʱ����Һ�е��������ӷֱ��������ƶ��������еķ�ɢ��������ijһ���ƶ�
C����Һ���������ӵ��˶��й��ɣ������з�ɢ�����ӵ��˶����ɣ��������˶�
D��һ�����߷ֱ�ͨ����Һ�ͽ���ʱ������������ԵĹ����ǰ����û��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ������ ��
A������ʽΪC4H10O�Ĵ�������ͭ���ͼ��������±���������Ϊȩ��ͬ���칹�干��4��
B��2���ȶ�����NaOH�Ҵ�����Һ���ȵķ�Ӧ������һ��������ͬ���칹��
C��3������3���һ������һ�ȴ�����5��
D������ʽΪC7H8O���л�������Ȼ�����Һ������ɫ��Ӧ��ͬ���칹�干��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ΪNO��NO2������Na2O2��Ӧ��ijС��ѧ��������ɣ��������Ϸ���Na2O2��NO2���������ԣ����ݻ��ϼ�����ԭ��������貢����̽����
�����.Na2O2������NO2
�����.NO2������Na2O2
��1��С���ͬѧ�������ͼʵ��װ�ã�����������ʵ�飺
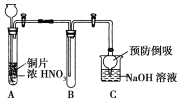
���Թ�A�з�����Ӧ�Ļ�ѧ����ʽ��______________________________��
�ڴ��Թ�B���ռ������壬���Թ�B�м�������Na2O2��ĩ���������ӣ��������Թ��ڷ�ĩ���۲쵽����ɫ����Ѹ����ʧ���ٽ��������ľ��Ѹ������Թ��ڣ�����ľ����ȼ����ͬѧ��Ϊ�������ȷ����ͬѧ��Ϊ��װ�ò��ܴﵽʵ��Ŀ�ģ�Ϊ�ﵽʵ��Ŀ�ģ���A��B֮������һ��װ�ã���װ�õ�������______________________________��
����ͬѧ�øĽ���װ�ã��ظ��˼�ͬѧ��ʵ��������۲쵽����ɫ����Ѹ����ʧ�����������ľ��δ��ȼ���ó����ۣ��������ȷ��NO2��Na2O2��Ӧ����ѧ����ʽ��
_____________________________________________________________________��
��2����ͬѧ��ΪNO�ױ�O2���������ױ�Na2O2������
�������ϣ���2NO��Na2O2===2NaNO2
��6NaNO2��3H2SO4===3Na2SO4��2HNO3��4NO����2H2O
�����������£�NO��NO������MnO��Ӧ����NO��Mn2��
��ͬѧ����ͼ��ʾװ��(���ּг�װ����)̽��NO��Na2O2�ķ�Ӧ��
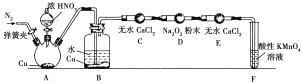
��Aװ����ʢװCuƬ������������__________��B�й۲쵽����Ҫ������______________________��Fװ�õ�������______________________________��
���ڷ�Ӧǰ�����ɼУ�ͨ��һ��ʱ��N2��Ŀ����______________________________��
�۳�ַ�Ӧ����Dװ���в�����NaNO2����NaNO3��ʵ�鷽����____________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�������ܱ������У���һ�������·�����Ӧ��2A������![]() B (g)+C(s)���Ҵﵽƽ�⣬�������¶�ʱ��������������ܶȱ���������жϴ������
B (g)+C(s)���Ҵﵽƽ�⣬�������¶�ʱ��������������ܶȱ���������жϴ������
A. ������ӦΪ���ȷ�Ӧ����AΪ����̬
B. ������ӦΪ���ȷ�Ӧ����AΪ��̬
C. ����ƽ����ϵ�м�������C�����ƽ�ⲻ�ƶ�
D. ѹǿ�Ը�ƽ����ƶ���Ӱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ij��Ӧ��ƽ�⣬ƽ�ⳣ��K=![]() ������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ����
������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ����
A���÷�Ӧ���ʱ�Ϊ��ֵ
B�����º����£�����ѹǿ��H2Ũ��һ����С
C�������¶ȣ��淴Ӧ���ʼ�С
D���÷�Ӧ��ѧ����ʽΪCO+H2O=CO2+H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶ȡ�ѹǿ�£�1���X2�����3���Y2���廯������2��������廯���������廯����Ļ�ѧʽΪ (����)
A. XY3 B. XY C. X3Y D. X2Y3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO2����Ҫ�Ļ���ԭ�ϣ�Ҳ��Ӧ�ù㷺�Ļ�����Ʒ��CO2��������ƻ������ط�Ӧ�ɲ���������
������м��㣺
��1��CO2ͨ�백ˮ����NH4HCO3��NH4HCO3�����ֽ⡣2.00mol NH4HCO3��ȫ�ֽ⣬�ֽ���ᆳ���������Ϊ_________L(��״��)��
��2��ijH2�к���2.40 molCO2���û������ͨ��2.00 L NaOH��Һ�У�CO2����ȫ���ա����NaOH��ȫ��Ӧ����NaOH��Һ��Ũ��Ϊ_______��
��3��CO2��KO2�����з�Ӧ��
4KO2+2CO2��2K2CO3+3O2
4KO2+4CO2+2H2O��4KHCO3+3O2
��9 mol CO2���ܷ���ں�KO2��Ӧ������9 molO2����Ӧǰ�ܷ����H2O����Ӧ���Ƕ��٣���ʽ���㡣
��4�������ˮ������Ӧ�IJ����Ǻϳɼ״���ԭ�ϣ�CH4+H2O![]() CO+3H2
CO+3H2
��֪��CO+2H2![]() CH3OH CO2+3H2
CH3OH CO2+3H2![]() CH3OH+H2O
CH3OH+H2O
300 mol CH4��ȫ��Ӧ��IJ����У�����100 mol CO2��ϳɼ״�������ü״�350 mol����������120 mol������CO2��ת���ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����û�ѧ��Ӧԭ��֪ʶ�о��������CO��SO2����Ⱦ������Ҫ���塣
��1����CO���Ժϳɼ״�����֪��
CH3OH(g)��3/2O2(g)===CO2(g)��2H2O(l) ��H����764.5 kJ��mol��1
CO(g)��1/2O2(g)===CO2(g) ��H����283.0 kJ��mol��1
H2(g)��1/2O2(g)===H2O(l) ��H����285.8 kJ��mol��1
��CO(g)��2H2(g) ![]() CH3OH(g) ��H��______kJ��mol��1��
CH3OH(g) ��H��______kJ��mol��1��
��2�����д�ʩ���ܹ����������ϳɼ״���Ӧ���ʵ���________(��д���)��
a��ʹ�ø�Ч���� b�����ͷ�Ӧ�¶�
c��������ϵѹǿ d�����Ͻ�CH3OH�ӷ�Ӧ������з������
��3����һ��ѹǿ�£��ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ
����ͼ��ʾ��
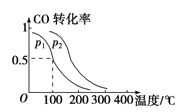
��p1________p2(����ڡ�����С�ڡ����ڡ�)��
��100 ��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��________��
���������������������£�������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת����________(���������С�����䡱)��
��4��ij����С����SO2Ϊԭ����ȡ���ᡣ
������ԭ���ԭ������SO2��O2��H2O���Ʊ����ᣬ�õ���ö�ײ������缫�������������壬ͬʱҲ��ʹ������������Һ��ֽӴ�����д���õ�ظ����ĵ缫��Ӧʽ��_____________________��
����Na2SO3��Һ�������SO2��NaHSO3��Һ��Ȼ�������Һ���Ƶ����ᡣ���ԭ��ʾ��ͼ���¡���д����ʼʱ������Ӧ�ĵ缫��Ӧʽ��_________________��
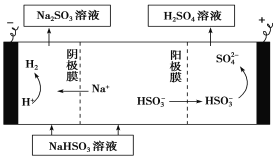
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com