
Ўҫ»ҜС§——СЎРЮ2Јә»ҜС§УлјјКхЎҝ(15·Ц)
»ҜС§КЗИЛАаҪшІҪөД№ШјьЈ¬»ҜС§ОӘИЛАаөДЙъІъЎўЙъ»оМṩБЛОпЦКұЈЦӨЎЈ
(1)өз¶ЖКұЈ¬УГ¶ЖІгҪрКфЧчСфј«өДЧчУГКЗ Ј®
ОӘБЛК№¶ЖІгәс¶ИҫщФИЎў№в»¬ЦВГЬЎўУл¶ЖјюөДёҪЧЕБҰЗҝЈ¬іэҝШЦЖИЬТәЦРАлЧУЕЁ¶ИНвЈ¬
НЁіЈ»№ҝЙТФІЙИЎөДҙлК©УР ЎЈ
(2)ВұЛ®ЦРФМә¬ЧЕ·бё»өДГҫЧКФҙЈ¬ҫӯЧӘ»ҜәуҝЙ»сөГMgCl2ҙЦІъЖ·ЎЈҙУВұЛ®ЦРМбИЎГҫөД
ІҪЦиОӘЈә
aЈ®Ҫ«әЈұЯҙуБҝҙжФЪөДұҙҝЗмСЙХіЙКҜ»ТЈ¬ІўҪ«КҜ»ТЦЖіЙКҜ»ТИйЈ»
bЈ®Ҫ«КҜ»ТИйјУИлөҪәЈЛ®іБөніШЦРҫӯ№эВЛөГөҪMg(OH)2іБөнЈ»
c.ФЪMg(OH)2іБөнЦРјУИлСОЛбөГөҪMgCl2ИЬТәЈ¬ФЩҫӯХф·ўҪбҫ§өГөҪMgCl2·6H2OЈ»
dЈ®Ҫ«MgCl2·6H2OФЪТ»¶ЁМхјюПВјУИИөГөҪОЮЛ®MgCl2Ј»
eЈ®өзҪвИЫИЪөДВИ»ҜГҫҝЙөГөҪMgЎЈ
ўЩІҪЦиdЦРөДЎ°Т»¶ЁМхјюЎұЦёөДКЗ Ј¬ДҝөДКЗ ЎЈ
ЙПКцМбИЎГҫөДБчіМЦР,ОӘБЛҪөөНіЙұҫЈ®јхЙЩОЫИҫЈ¬ҝЙТФІЙИЎәЬ¶аҙлК©Ј¬ЗлРҙіцЖдЦРТ»өг
ўЪУРН¬С§ИПОӘЈәІҪЦиbәуҝЙјУИИMg(OH)2өГөҪMgOЈ¬ФЩөзҪвИЫИЪөДMgOЦЖҪрКфГҫЈ¬ХвСщҝЙјт»ҜКөСйІҪЦиЈ¬ДгН¬ТвёГН¬С§өДПл·ЁВр?ОӘКІГҙ?
(3)УЛКЗәЛ·ҙУҰЧоЦШТӘөДИјБПЈ¬ТСҫӯСРЦЖіЙ№ҰТ»ЦЦу§әПРНАлЧУҪ»»»КчЦ¬Ј¬ЛьЧЁГЕОьёҪәЈЛ®ЦР өДU4+Ј¬¶шІ»ОьёҪЖдЛыФӘЛШЎЈЖд·ҙУҰФӯАнОӘ (КчЦ¬УГHRҙъМж)Ј¬·ўЙъАлЧУҪ»»»әуөДАлЧУҪ»»»ДӨУГЛбҙҰАн»№ҝЙФЩЙъІўөГөҪә¬УЛөДИЬТәЈ¬Жд·ҙУҰФӯАнОӘЈә ЎЈ
(4)°ўЛҫЖҘБЦ(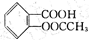 )ФЪіұКӘҝХЖшЦРҝЙ·ЦҪвіЙЛ®СоЛбәНҙЧЛб¶шВФҙшЛбіфО¶Ј¬№КГЬ·вұЈҙжЈ¬УГ»ҜС§·ҪіМКҪұнКҫ°ўЛҫЖҘБЦұШРлЦьІШУЪГЬұХЎўёЙФпҙҰөДФӯТтЈә
)ФЪіұКӘҝХЖшЦРҝЙ·ЦҪвіЙЛ®СоЛбәНҙЧЛб¶шВФҙшЛбіфО¶Ј¬№КГЬ·вұЈҙжЈ¬УГ»ҜС§·ҪіМКҪұнКҫ°ўЛҫЖҘБЦұШРлЦьІШУЪГЬұХЎўёЙФпҙҰөДФӯТтЈә
Ј¬ҙЛ·ҙУҰөДАаРНКфУЪ ЎЈ
Ўҫ»ҜС§——СЎРЮ2Јә»ҜС§УлјјКхЎҝЈЁ15·ЦЈ©
(1)¶ЖІгҪрКфФЪСфј«К§ИҘөзЧУЈ¬ј°КұІ№ідИЬТәЦРөДАлЧУЈ¬К№ИЬТәЦРАлЧУЕЁ¶ИұЈіЦІ»ұдЈ¬ҙУ¶шК№өз¶ЖөДЛЩ¶ИұЈіЦІ»ұдЈ¬К№¶ЖІгәс¶ИҫщФИ (2·Ц)ККөұҪөөНөз¶ЖКұЦұБчөзФҙөДөзС№ЎўФЪөз¶ЖТәЦРјУИлЙЩБҝөДұнГж»оРФјБ(2·Ц)
(2)ўЩФЪHClЖшБчЦР(1·Ц) ТЦЦЖMgCl2Л®Ҫв(1·Ц) ўЪөзҪвІъЙъөДC12УГУЪЦЖИЎHClЖшМе(1·Ц)
ўЫІ»Н¬ТвЈ¬ТтОӘMgOИЫөгәЬёЯЈ¬ИЫИЪКұТтәД·СҙуБҝөДДЬБҝ¶шФцјУЙъІъіЙұҫ(3·Ц)
(3)4HR+U4+=UR4+4H+ (1·Ц) UR4+4H+=4HR+U4+ (1·ЦЈ©
(4)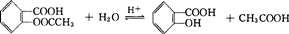 (2·Ц) Л®Ҫв·ҙУҰ(»тИЎҙъ·ҙУҰ)(1·Ц)
(2·Ц) Л®Ҫв·ҙУҰ(»тИЎҙъ·ҙУҰ)(1·Ц)



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
»ҜС§ЙПҙжФЪРн¶аөДөЭұд№ШПөЈ¬ПВБРөЭұд№ШПөНкИ«ХэИ·өДКЗЈЁ Ј©
AЈ®АлЧУ°лҫ¶ЈәNa+>Mg2+>Al3+>ClЎӘ Ј»ФӯЧУ°лҫ¶ЈәNa>Mg>Al>Cl
BЈ®ОИ¶ЁРФЈәHF>H2O>NH3>CH4 Ј» »№ФӯРФЈә HF < H2O < NH3< CH4
CЈ®јоРФЈәCsOH>KOH>Mg(OH)2>NaOH Ј» ҪрКфРФЈәCs>K>Mg>Na
DЈ®ЛбРФЈәHClO>H2SO4>H2CO3Ј»·ЗҪрКфРФCl>S>C
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ДЬТ»ҙОЗш·ЦCH3CH2OHЎўCH3COOHЎў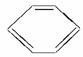 әНCCl4ЛДЦЦОпЦКөДКФјБКЗ
әНCCl4ЛДЦЦОпЦКөДКФјБКЗ
(ЎЎЎЎ)ЎЈ
AЈ®H2O BЈ®NaOHИЬТә
CЈ®СОЛб DЈ®КҜИпКФТә
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
·ПҫЙЗҰРоөзіШөД»ШКХАыУГКЗ·ўХ№Сӯ»·ҫӯјГөДұШҫӯЦ®В·ЎЈЖдТхЎўСфј«МоідОпЈЁЗҰёаЈ¬ЦчТӘә¬PbOЎўPbO2ЎўPbSO4Ј©КЗ·ПҫЙЗҰРоөзіШөДЦчТӘІҝ·ЦЈ¬»ШКХКұЛщөГ»ЖөӨЈЁPbOЈ©ЎўМјЛбЗҰҝЙУГУЪәПіЙИэСО»щБтЛбЗҰЈЁЧйіЙҝЙұнКҫОӘ3PbOЎӨPbSO4ЎӨH2OЈ©Ј¬Жд№ӨТХБчіМИзПВЈә
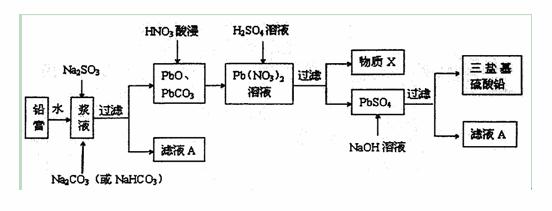
ЈЁ1Ј©УГМјЛбСОЧчЧӘ»ҜјБЈ¬Ҫ«ЗҰёаЦРөДБтЛбЗҰЧӘ»ҜОӘМјЛбЗҰЈ¬РҙіцЧӘ»Ҝ·ҙУҰ·ҪіМКҪЈә
ЈЁ2Ј©ВЛТәAДЬУГАҙ»ШКХNa2SO4ЎӨ10H2OЈ¬МбИЎёГҫ§МеөДЦчТӘІҪЦиУР____________Ўў___________Ўў№эВЛЎўПҙөУЎўёЙФпЈ»јмСйёГҫ§МеЦРТхАлЧУөДКөСй·Ҫ·ЁКЗ________ ____ЎЈ
ЈЁ3Ј©ОпЦКXКЗТ»ЦЦҝЙСӯ»·К№УГөДОпЦКЈ¬ЖдИЬЦКЦчТӘКЗ______________ЈЁМо»ҜС§КҪЈ©Ј¬ИфXЦРІРБфөД 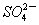 №э¶аЈ¬Сӯ»·АыУГКұҝЙДЬіцПЦөДОКМвКЗ__________________ЎЈ
№э¶аЈ¬Сӯ»·АыУГКұҝЙДЬіцПЦөДОКМвКЗ__________________ЎЈ
ЈЁ4Ј©ЙъіЙИэСО»щБтЛбЗҰөДАлЧУ·ҪіМКҪОӘ____________________ЎЈ
ЈЁ5Ј©ПтЗҰёаҪ¬ТәЦРјУИлNa2SO3ИЬТәөДДҝөДКЗҪ«ЖдЦРөДPbO2»№ФӯОӘPbOЎЈИфКөСйЦРЛщИЎЗҰёаөДЦКБҝОӘ47Ј®8gЈ¬ЖдЦРPbO2өДЦКБҝ·ЦКэОӘ15ЈҘЈ¬ФтТӘҪ«PbO2И«Іҝ»№ФӯЈ¬ЦБЙЩРиТӘјУИл______mLөД0.5 mol/LNa2SO3ИЬТәЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
УГNa2SO3ИЬТәОьКХБтЛб№ӨТөОІЖшЦРөД¶юСх»ҜБтЈ¬Ҫ«ЛщөГөД»мәПТәҪшРРөзҪвСӯ»·ФЩЙъЈ¬ХвЦЦРВ№ӨТХҪРФЩЙъСӯ»·НСБт·ЁЎЈЖдЦРТхЎўСфАлЧУҪ»»»ДӨЧйәПСӯ»·ФЩЙъ»ъАнИзПВНјЛщКҫЈ¬ФтПВБРУР№ШЛө·ЁЦРІ»ХэИ·өДКЗ
AЈ®XОӘЦұБчөзФҙөДёәј«Ј¬YОӘЦұБчөзФҙөДХэј«
BЈ®Сфј«ЗшpHФцҙу
CЈ®НјЦРөДb>a
DЈ®ёГ№эіМЦРөДІъЖ·ЦчТӘОӘH2SO4әНH2
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ПВБРУР№ШЛө·ЁҙнОуөДКЗ
AЈ®Лі-2-¶ЎП©әН·ҙ-2-¶ЎП©·ЦұрУлH2јУіЙЈ¬ЙъіЙПаН¬өДјУіЙІъОп
BЈ®өИЦКБҝөДТТНйЎўТТП©ЎўТТИІНкИ«ИјЙХәДСхБҝТАҙОјхРЎ
CЈ®ТТНйөДЛДВИҙъОпУР2ЦЦ
DЈ®(CH3)3CClәН(CH3)2CHCH2Cl·ЦұрУлNaOHөДТТҙјИЬТә№ІИИЈ¬ЙъіЙІ»Н¬өДПыИҘІъОп
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
Al3Ј«І»ДЬәН______________________________________________ҙуБҝ№ІҙжЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
»ҜС§УлЙъ»оГЬЗРПа№ШЈ¬ПВБРЛө·ЁІ»ХэИ·өДКЗЈә
A.ТТП©ҝЙЧчЛ®№ыөДҙЯКмјБ
B.№иҪәҝЙЧчҙьЧ°КіЖ·өДёЙФпјБ
C.СҮ¶ыВнБЦҝЙЧчКіЖ·өДұЈПКјБ
D.ЗвСх»ҜВБҝЙЧчОёЛбөДЦРәНјБ
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com