
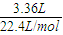 =0.15mol����Ӧ�������ӵ����ʵ���Ϊ0.15×2=0.3mol��
=0.15mol����Ӧ�������ӵ����ʵ���Ϊ0.15×2=0.3mol�� H2��3HCl��Al3+��3NaOH��Al��OH��3
H2��3HCl��Al3+��3NaOH��Al��OH��3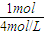 =0.25L=250mL��
=0.25L=250mL��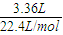 ×2×17g/mol+ag=��a+5.1��g��
×2×17g/mol+ag=��a+5.1��g��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�
ij�о���ѧϰС��Ϊ�ⶨþ�����Ļ����������������������ȡ�û����a g������200 mL 5 mol/L HCl��Һ�У����ռ�����״���µ�����3.36 L��
��1��a ��ȡֵ��Χ�� ��
��2����Ӧ��������Һ������4 mol/L��NaOH��Һ�������������ﵽ���ֵʱ�������NaOH��Һ������� mL����������������� g�����ú�a �Ĵ���ʽ��ʾ��
��3����������NaOH��Һ���������ٷ����仯ʱ�����ˡ�ϴ�Ӳ����ճ��������أ����ò�������������Ϊa g��ԭ������������������� ����д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��2012ѧ��Ⱥ���������ʵ����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��8�֣�
ij�о���ѧϰС��Ϊ�ⶨþ�����Ļ����������������������ȡ�û����a g������200 mL 5 mol/L HCl��Һ�У����ռ�����״���µ�����3.36 L��
��1��a ��ȡֵ��Χ�� ��
��2����Ӧ��������Һ������4 mol/L��NaOH��Һ�������������ﵽ���ֵʱ�������NaOH��Һ������� mL����������������� g�����ú�a �Ĵ���ʽ��ʾ��
��3����������NaOH��Һ���������ٷ����仯ʱ�����ˡ�ϴ�Ӳ����ճ��������أ����ò�������������Ϊa g��ԭ������������������� ����д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��Ⱥ���������ʵ����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��8�֣�
ij�о���ѧϰС��Ϊ�ⶨþ�����Ļ����������������������ȡ�û����a g������200 mL 5 mol/L HCl��Һ�У����ռ�����״���µ�����3.36 L��
��1��a ��ȡֵ��Χ�� ��
��2����Ӧ��������Һ������4 mol/L��NaOH��Һ�������������ﵽ���ֵʱ�������NaOH��Һ������� mL����������������� g�����ú�a �Ĵ���ʽ��ʾ��
��3����������NaOH��Һ���������ٷ����仯ʱ�����ˡ�ϴ�Ӳ����ճ��������أ����ò�������������Ϊa g��ԭ������������������� ����д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ĩ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com