
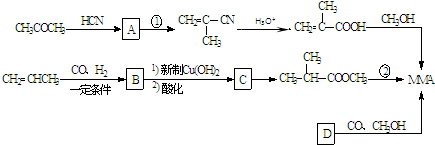
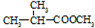 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��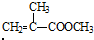 +CH3CH2CH2OH��
+CH3CH2CH2OH��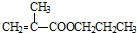 +CH3OH
+CH3OH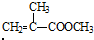 +CH3CH2CH2OH��
+CH3CH2CH2OH��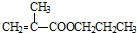 +CH3OH
+CH3OH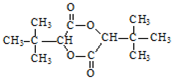 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��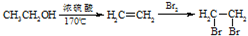
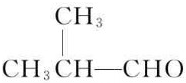 ��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��
��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��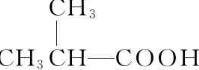 ��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��������ʵĽṹ���������������
��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��������ʵĽṹ��������������� ��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��
��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��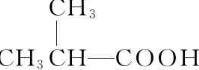 ��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��
��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��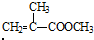 +CH3CH2CH2OH��
+CH3CH2CH2OH��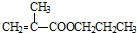 +CH3OH��
+CH3OH��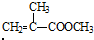 +CH3CH2CH2OH��
+CH3CH2CH2OH��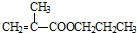 +CH3OH��
+CH3OH��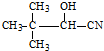 ��
��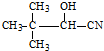 ��Ũ���������·�Ӧ����
��Ũ���������·�Ӧ����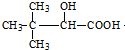 ��
��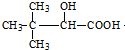 �������Ӽ�������Ӧ����
�������Ӽ�������Ӧ����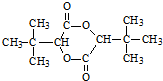 ��
�� ��
��

 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com