��2012?���գ����ǵؿ��к�����ߵĽ���Ԫ�أ��䵥�ʼ��Ͻ������������е�Ӧ�������㷺��
��1�����̼�Ȼ�ԭ-�Ȼ�����ʵ�����������Ʊ�������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
Al
2O
3��s��+AlC
13��g��+3C��s��=3AlCl��g��+3CO��g����H=a kJ?mol
-13AlCl��g��=2Al��l��+AlC
13��g����H=b kJ?mol
-1�ٷ�ӦAl
2O
3��s��+3C��s��=2Al��l��+3CO��g���ġ�H=
a+b
a+b
kJ?mol
-1���ú�a��b �Ĵ���ʽ��ʾ����
��Al
4C
3�Ƿ�Ӧ�����е��м���Al
4C
3 �����ᷴӦ������֮һ�Ǻ�������ߵ����� �Ļ�ѧ����ʽΪ
Al4C3+12HCl=4AlCl3+3CH4��
Al4C3+12HCl=4AlCl3+3CH4��
��
��2��þ���Ͻ�Mg
17Al
12 ����һ��DZ�ڵ�������ϣ�������������£���һ����ѧ�����ȵ�Mg��Al ������һ���¶���������ã��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪMg
17Al
122+17H
2=17MgH
2+12Al���õ��Ļ����Y��17MgH
2+12Al����һ�������¿��ͷų�������
�������Ʊ�þ���Ͻ�Mg
17Al
12��ʱͨ�������Ŀ����
��ֹMg Al����������
��ֹMg Al����������
��
����6.0mol?L-1HCl ��Һ�У������Y ����ȫ�ͷų�H
2��1mol Mg
17 Al
12 ��ȫ�����õ��Ļ����Y ������������ȫ��Ӧ���ͷų�H
2 �����ʵ���Ϊ
52mol
52mol
��
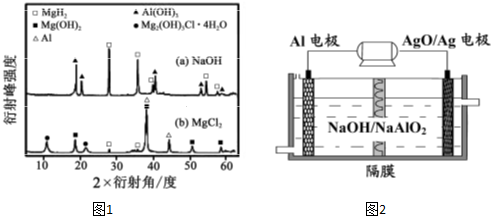
����0.5mol?L-1NaOH ��1.0mol?L
-1 MgCl
2��Һ�У������Y ��ֻ�ܲ��ַų���������Ӧ������������ʵ�X
-����������ͼ��ͼ1��ʾ��X
-��������������ж�ij��̬��
���Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ����������NaOH ��Һ�У������Y �в�����������Ҫ������
Al
Al
�˿�
�˿�
���ѧʽ����
��3�������������Խ��Al-AgO ��ؿ�����ˮ�¶�����Դ����ԭ����ͼ2��ʾ���õ�ط�Ӧ�Ļ�ѧ����ʽΪ
2Al+3AgO+2NaOH=2NaAlO2+3Ag+H2O
2Al+3AgO+2NaOH=2NaAlO2+3Ag+H2O
��




