
����Ŀ��ij�л���A����Է�������Ϊ62��Ϊ��һ���ⶨA �Ļ�ѧʽ��ȡ3.1g A��ȫȼ�գ��õ�������̼��ˮ�������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����2.7 g��4.4 g������ÿ����Ӧ��ȫ����
��1�����л����ʵ��ʽ��__________________������ʽ��________________��
��2�����������ʾ�С�C��C�����͡�O��H�����������գ����˴Ź�������ֻ��2�����շ��ҷ����֮��Ϊ1��2���ƶϸ��л���Ľṹ��ʽ��__________________��
��3�����л���������Ʒ�Ӧ�Ļ�ѧ����ʽ��____________________��
���𰸡� CH3O C2H6O2 CH2OHCH2OH CH2OHCH2OH+2Na![]() CH2ONaCH2ONa+H2
CH2ONaCH2ONa+H2
���������������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����2.7 g��4.4 g�����ӵ������ֱ���ˮ�Ͷ�����̼�������������л���A�����ԭ��������������غ㶨���ж�ʵ���ҡ�����ʽ�������л����������д�ṹ��ʽ��
��⣺��1��3.1gA�����ʵ�����0.05mol�����ɵ�ˮ��2.7g�����ʵ�����2.7g��18g/mol��0.15mol��������ԭ�ӵ����ʵ�����0.3mol�����ɵ�CO2��4.4g�����ʵ�����4.4g��44g/mol��0.1mol������̼ԭ�ӵ����ʵ�����0.1mol�����Ը���ԭ���غ��֪��A�����к��е�̼����ԭ�Ӹ����ֱ���2����6��������ԭ�ӵĸ�����![]() ��������A�Ļ�ѧʽ��C2H6O2�����ʽ��CH3O��
��������A�Ļ�ѧʽ��C2H6O2�����ʽ��CH3O��
��2�����ݺ��������ʾ�С�C��C�����͡�O��H�����������գ��˴Ź�������ֻ��2�����շ��ҷ����֮��Ϊ1��2��֪�����л������Ҷ������ṹ��ʽ��HOCH2CH2OH��
��3�����ܺͽ����Ʒ�Ӧ������������÷�Ӧ�Ļ�ѧ����ʽ��HOCH2CH2OH��2Na��NaOCH2CH2ONa��H2����


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ֶ����ڽ���Ԫ�غ�һ�ַǽ���Ԫ����ɵĻ�����X����ˮ�������ֽⷴӦ��ijУ��ȤС������ͼװ��(�г�װ����ȥ)�������̽��ʵ�顣
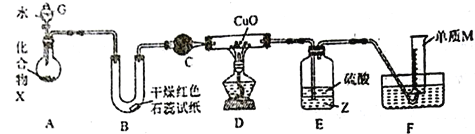
(1)����G��������_____________________��B�к�ɫʯ����ֽ����������M�ĵ���ʽΪ___________________��
(2)������X�к���ɵ���M��Ԫ����������Ϊ16.9%��д��X��ˮ��Ӧ�Ļ�ѧ����ʽ��_____________________________��
(3)C�е��Լ�����Ϊ___________________________��
(4)ʵ��ʱ��װ��D��Ӳ�ʲ������ڵ�����Ϊ_________________________��
(5)�b��E���Լ�ZΪ___________(�ѧʽ)��װ��E��������_________________________��
(6)����ͨ��E��F��װ�ã������ʵ�鷽��֤��D�з����˷�Ӧ(��ͨ���۲�D�й�����ɫ�����仯)��__________________________________________________��
(7)��װ��A�й�����Ʒ��������(���ʲ����뷴Ӧ)��ijͬѧͨ���ⶨF�е���M�ڱ�״���µ����������Ʒ����������ȷ��������Ʒ��X�������������жϸ÷����Ƿ���У���˵��ԭ��__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Ge)�ǵ��͵İ뵼��Ԫ�أ��ڵ��ӡ����ϵ�����Ӧ�ù㷺���ش��������⣺
(1)��̬Geԭ�ӵĺ�������Ų�ʽΪ[Ar]________����________��δ�ɶԵ��ӡ�
(2)Ge��C��ͬ��Ԫ�أ�Cԭ��֮������γ�˫������������Geԭ��֮�������γ�˫������������ԭ�ӽṹ�Ƕȷ�����ԭ����_______________________________��
(3)�Ƚ�������±������۵�ͷе㣬������仯���ɼ�ԭ��_____________________��
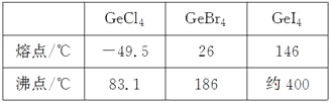
(4)�����ԭCO2�Ʊ�CH4��Ӧ�У���״����Zn2GeO4�Ǹ÷�Ӧ�����ô�����Zn��Ge��O�縺���ɴ���С��˳����________________��
(5)Ge�������н��ʯ�ͽṹ������Geԭ�ӵ��ӻ���ʽΪ____________����֮����ڵ���������________________��
(6)��������������Ҫ�أ�
��ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�ã���ͼΪGe�����ľ���������ԭ���������AΪ(0,0,0)��BΪ(1/2��0��1/2)��CΪ(1/2��1/2��0)����Dԭ�ӵ��������Ϊ________��
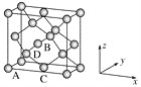
�ھ������������������Ĵ�С����״����֪Ge�����ľ�������a��565.76 pm�����ܶ�Ϊ________g��cm��3(�г�����ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ˮ����õ���ˮ��Ƚϣ����н��۲���ȷ����
A. ��ɫ��ͬB. ǰ����ʹ��ɫ������ɫ
C. ������H+D. ��AgNO3��Һ�������ɰ�ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڷ�Ӧ2KMnO4��16HCl===2KCl��2MnCl2��5Cl2����8H2O�У�����˵����ȷ����(����)
A. ���������뻹ԭ�������ʵ���֮����2��5
B. ��������HClռ�μӷ�ӦHCl�ܷ�������![]()
C. KMnO4ֻ��һ���ַ�����ԭ��Ӧ
D. KMnO4��HClǡ�÷ֱ���ȫ������ԭ��Ӧ��������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����������ȵĻ��������������ȷ����( )
A.������������������Ӧ����FeCl3B.����SO2ͨ��Ca(ClO)2��Һ����CaSO3����
C.������ʹ��ʪ�ĵ��۵⻯����ֽ����D.������Ʊȴ������ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�б�����뻹ԭ�����ܽ��е���
A. KMnO4�� MnO2
B. Zn�� Zn2��
C. Cl2��Cl��
D. CuO��Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
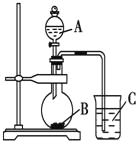
����Ŀ����ұ���ķ���������Ϊԭ����ȡ��ϸ�����������Ƚ��ͻ�����Ⱦ�ֿ��������Դ�������ʡ���֪���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3�������Ʊ�ʵ����������ͼ��
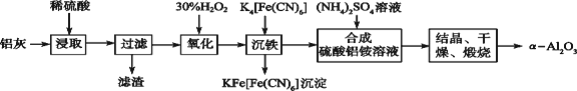
��1��д������������������Һ��Ӧ�漰�Ļ�ѧ����ʽ��_______________________��
��2����30%H2O2��Һ���������ӷ�Ӧ����ʽΪ______________________________��
��3������������茶��壬��������Ҫ��ӦΪ��4[NH4Al(SO4��2��12H2O]![]() 2Al2O3��2NH3����N2����5SO3����3SO2����53H2O��������������ͨ����ͼ��ʾ��װ�á�
2Al2O3��2NH3����N2����5SO3����3SO2����53H2O��������������ͨ����ͼ��ʾ��װ�á�
����ƿ���ռ�����������_______���ѧʽ����
������KMnO4��Һ��ɫ��dz_______����ܡ����ܡ���˵������������茶�������к���SO2���壿���ɣ�______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
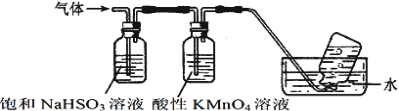
����Ŀ��ij�о���ѧϰС�����ʵ����֤���¹��ɣ�Ԫ�صķǽ�����Խǿ����Ӧ������������ˮ�������Ծ�Խǿ����С���������ͼװ�ý���ʵ�顣��Ƚ�C��Si��S����Ԫ�صķǽ����ԡ�
(1)�Ʋ�����Ԫ�ض�Ӧ������������ˮ����������ǿ������˳����_________________(�ѧʽ)��
(2)��B��C��Ϊ���Σ�����ɫ��Ӧ��Ϊ��ɫ��A��B��C�Ļ�ѧʽ����Ϊ_________��__________��_________(A��C�����ʵĻ�ѧʽ)��
(3)��ƿ�е�ʵ������Ϊ________________________________��
(4)����BҲ������B����Һ���档ʵ��������0.1mol/L��B����Һ450mL����Ҫ��������ƽ��ȡB_____g��
(5)��ͬѧ��Ϊ����A�����ʻ�ΪŨ���ᣬ�����Լ����䡣�������֤N��C��Si��Ԫ�صķǽ�����ǿ��������Ϊ����ͬѧ�Ĺ۵�___________(ѡ������ȷ����������)��������__________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com