
 �ã�ã�����
�ã�ã����� �ã��������ã�������������
�������������������� �����
����� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��Ϊ17g����H2R��Ħ�������� ����������NH3��H2R�����ʵ�����Ϊ ��1.7g������ mol H2O���еĵ�������ȡ�
��Ϊ17g����H2R��Ħ�������� ����������NH3��H2R�����ʵ�����Ϊ ��1.7g������ mol H2O���еĵ�������ȡ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
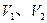 ��
��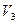 ����
����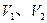 ��
��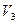 �Ĵ�С��ϵ��ȷ���ǣ�������
�Ĵ�С��ϵ��ȷ���ǣ�������A��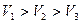 |
B��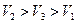 |
C��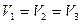 |
D��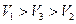 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������ǻ�ԭ�� |
| B��ά����C��Fe3+��ԭΪFe2+ |
| C������C�������� |
| D���������α���ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������Ͷ��ˮ�л�����ը |
| B�����һ��ǿ��ԭ��������Ӻ��ȶ� |
| C������Ⱥ����ڿ����о���ȼ�գ����ɱȹ�����������ӵ������� |
| D���ԭ�ӵĺ˵�����ȼ�ԭ�ӵĺ˵�����࣬����ԭ��ʧ���ӵ�����С�ڼ�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ԫ�ص����� | B�����ʵ����� |
| C�����ӵ����� | D������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���٢ڢ� | C���٢ۢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��a="b" | B��a��b |
| C��a��b | D�����Ƚ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2CO3������ | B��NaOH������ | C��Na2O���� | D��NaHCO3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com