
��������ʵ��װ�òⶨͭ������ͭ�������ͭԪ�صĺ�����
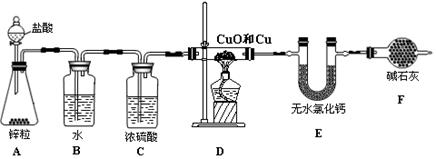
�Իش��������⣺
��1��A��ʢװ�������������Ϊ ��
��2��A�пɹ۲쵽������Ϊ ��
��3��Bװ�õ������� ��
��4���ڸ�Dװ�ü���ǰ��Ӧ�ý��е�һ�������� ����Ŀ���ǣ�
��
��5����֪Dװ����ͭ������ͭ����������Ϊ10 g������������Ӧǰ��Eװ�õ������ֱ�Ϊ100.2 g��102.0 g��ԭ�������ͭԪ�ص���������Ϊ (�����װ���еķ�Ӧ�����ն�����ȫ��)��
��6���粻��Fװ�ã���ʹʵ���� (�ƫ�͡���ƫ�ߡ�)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
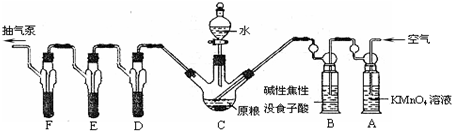
 �����������Ҫ���о���Ӧ�ü�ֵ��
�����������Ҫ���о���Ӧ�ü�ֵ��| ���� |
| ��� | ���� | ���� | ���� | |
| �¶�/�� | ���� | |||
| 1 | 40 | FeCl3��Һ | ||
| 2 | 20 | FeCl3��Һ | ||
| 3 | 20 | MnO2 | ||
| 4 | 20 | �� | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ӱ�ʡ�е�����У������ѧ����ĩ������ѧ�Ծ� ���ͣ�ʵ����
��������ʵ��װ�òⶨͭ������ͭ�������ͭԪ�صĺ�����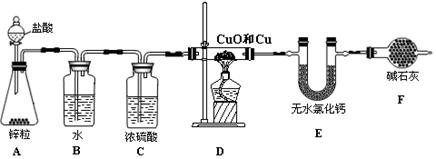
�Իش��������⣺
��1��A��ʢװ�������������Ϊ ��
��2��A�пɹ۲쵽������Ϊ ��
��3��Bװ�õ������� ��
��4���ڸ�Dװ�ü���ǰ��Ӧ�ý��е�һ�������� ����Ŀ���ǣ�
��
��5����֪Dװ����ͭ������ͭ����������Ϊ10 g������������Ӧǰ��Eװ�õ������ֱ�Ϊ100.2 g��102.0 g��ԭ�������ͭԪ�ص���������Ϊ (�����װ���еķ�Ӧ�����ն�����ȫ��)��
��6���粻��Fװ�ã���ʹʵ���� (�ƫ�͡���ƫ�ߡ�)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com