
��08����һ����ģ����17�֣�ʵ������������װ�ã��̶�װ�á��������������ԣ������йذ�����ȡ��ʵ��̽�������ù����Լ��У�NH4Cl��NaCl��(NH4)2SO4��Ca(OH)2��CaO��NaOH��Һ���Լ��У�Ũ��ˮ��Ũ���ᡣ
 ������и��⣺
������и��⣺
��1�� ����װ�â���ȡNH3���䷴Ӧ�Ļ�ѧ����ʽΪ___________________________��Ҫ�ⶨ���ɵ�NH3�������������ѡ���װ����________________����װ����ţ���������ʢ�Լ�Ӧ���е������ǣ�___________________________________________
_______________________________________��
��2�� ����װ�â���ȡ���ռ������NH3����ƿ�ڵ��Լ�Ӧ��_____________����Һ©���е��Լ�Ӧ��______________���ռ�װ��Ӧѡ��________________����װ����ţ���֤���������ռ����IJ����ǣ�____________________________________________��
��3�� �������и����Լ���ϣ�����������ͬ��������ȡ�����ĶԱ�ʵ�飬��������������mL������״���£����±���
| 5.4g NH4Cl(s) | 5.4g (NH4)2SO4(s) |
6.0g Ca(OH)2(s,����) | ��1344 | ��1364 |
6.0g NaOH(s,����) | ��1568 | ��1559 |
6.0g CaO(s,����) | ��1753 | ��1792 |
�ӱ������ݷ�����ʵ������ȡ�����IJ�����ߵ���_____________������ţ�����ԭ����__________________________________________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08����һ����ģ����֪ij�л���Ľṹ��ʽ��ͼ�����ڸ��л����˵����ȷ���ǣ���
�� 1mol���л����������3mol�������Ʒ�����Ӧ
�� 1mol���л����������1mol̼�����Ʒ�����Ӧ
�� 1mol���л�����Ũ��ˮ��Ӧ���������3mol Br2
�� ���л����ڿ������ױ�����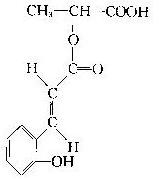
A���٢ڢ� B���٢ۢ� C���ڢۢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08����һ����ģ�������й�ʵ��IJ���������ȷ���ǣ���
A������ˮ�ķ�Ӧ�������Ӵ��Լ�ƿ��ȡ�������ƣ���С�������̶���С��һ���ƣ�С�ķ���װ��ˮ���ձ���
B������100mL��������Ϊ10%��H2O2��Һ��ȡһ֧���Ϊ100mL����Ͳ����ȡ��������Ϊ30%��˫��ˮ��Һ33.3mL��Ȼ���ˮ��100mL�̶���
C������ijdz��ɫ��Һ�к���Fe2+���ӣ�ȡ����Һ�����������еμ�KSCN��Һ�������ɫ���������еμ���ˮ����Һ��Ϊ��ɫ
D��ȡ����Һ©����������ϲ�Һ�壺���²�Һ��ӷ�Һ©���¶˹ܿ�������ʱ�رջ�����Ȼ�����һ��������������������Һ©���е�Һ��ų�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08����һ����ģ�����и�����������������ǰ��һ�����ں��ߵ��ǣ� ��
��HF��HBr�ķе� �ڴ�ˮ��25���80��ʱ��pH ��H+��H�������Ӱ뾶��25��ʱ���������pH������3�������AlCl3��Һ����ˮ���������H+������NH3+HNO3��N2+H2O��Ӧ�У�����ԭ�ͱ�������Nԭ���������ʵ���OH���ͨDOH�����ĵ�����
A���٢ڢ� B���ڢۢ� C���٢ڢ� D���ܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08����һ����ģ����14�֣�X��Y��Z��W��ԭ��������������Ķ�����Ԫ�أ�����ͬ�壬����ֻ������Ϊ����Ԫ�أ�Xԭ�ӵ������������������������ȣ�X��W��Y��Z������ԭ�ӵ�����������֮�;�Ϊ9��Y��W�ĵ��ʶ�����NaOH��Һ��Ӧ����ش��������⣺
��1��Y��Z��W��ԭ�Ӱ뾶��С�����˳����____________________����Ԫ�ط��ű�ʾ����
��2��Z��W��һ�����������γɻ�����ZW2�������и�ԭ�Ӿ��ﵽ��8�����ȶ��ṹ����ZW2�ĵ���ʽ��_______________�����ڳ����³�Һ̬���γɾ���ʱ������____________���塣
��3����ҵ����Y�ĵ��ʵ�ԭ����_____________________________���û�ѧ����ʽ��ʾ����
��4��X�ĵ�����Y�ĵ��ʻ�ѧ�������ƣ���X�ĵ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��________________________________________________��ע���������У�����XԪ�صĻ�����Ļ�ѧʽ�к���2����ԭ�ӣ�
��5��0.1molW�ĵ�����50mL 1.5mol?L��1��FeBr2��Һ��Ӧ����������Fe2+��Br�������ʵ���֮����____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com