
25�棬��c(CH3COOH)��c(CH3COO��)=0.1 mol/L��һ�����ʹ����ƻ����Һ����Һ��c(CH3COOH)��c(CH3COO��)��pHֵ�Ĺ�ϵ��ͼ��ʾ���й�����Ũ�ȹ�ϵ������ȷ����
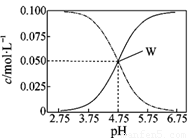
A��pH=5.5��Һ�У�c(CH3COO��) ��c(CH3COOH)��c(H��)��c(OH��)
B��W���ʾ��Һ�У�c(Na��)��c(H��)=c(CH3COO��)��c(OH��)
C��pH=3.5��Һ�У�c(Na��)��c(OH��)��c(CH3COOH)=0.1 mol/L
D����W������ʾ��Һ��ͨ��0.05molHCl����(��Һ����仯�ɺ���)��c(H��)=c(CH3COOH)��c(OH��)
AB
��������
���������A����ͼ��֪��pH=5.5 ����Һ�������ԣ���c��CH3COO-����c��CH3COOH�����������Ӵ����������ӣ���c��CH3COOH����c��CH3COO-����c��H+����c��OH-������A��ȷ��B��W���ɵ���غ��֪��c��Na+��+c��H+��=c��CH3COO-��+c��OH-������B��ȷ��C���ɵ���غ㼰c��CH3COOH��+c��CH3COO-��=0.1mol•L-1��֪��c��Na+��+c��H+��-c��OH-��+c��CH3COOH��==0.1mol/L����C����D��W��Ϊ�����Ĵ���ʹ����ƵĻ��Һ��1.0 L ��Һ��ͨ��0.05 mol HCl ����õ�0.05molNaCl��0.1molHAc���ɵ���غ��֪��c��H+��=c��CH3COO-��+c��OH-������D����ѡAB��
���㣺������Ҫ��������Ũ�ȴ�С�ıȽϣ���ȷ����ˮ�⡢�����غ��ǽ����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ŨH2SO4 |
| �� |
| �������� |

| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ϡ���� |
| �� |
| ϡ���� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� �� | ������ | �Ȼ��� | �廯�� | �⻯�� |
| �� | �� | �� | �� | |
| CH3- | -78.4 | -24.2 | 3.6 | 42.4 |
| CH3-CH2- | -37.7 | 12.3 | 38.40 | 72.3 |
| CH3-CH2-CH2- | 2.5 | 46.60 | 71.0 | 102.4 |
| ��CH3��2-CH- | -9.4 | 35.7 | 59.48 | 89.14 |
| CH3-CH2-CH2-CH2- | 32.5 | 78.44 | 101.6 | 130.5 |
| ��CH3��2-CH CH2- | 16.0 | 68.7 | 91.7 | 120.4 |
| ��CH3��3C- | 12.1 | 52 | 73.25 | 120.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶�/�� | 250 | 310 | 350 |
| K | 2.041 | 0.250 | 0.012 |
| Ũ��mol/L ʱ��/min |
c��CO�� | c��H2�� | c��CH3OH�� |
| 0 | 0.8 | 1.6 | 0 |
| 2 | 0.6 | 1.2 | 0.2 |
| 4 | 0.3 | 0.6 | 0.5 |
| 6 | 0.3 | 0.6 | 0.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��10�֣��л���Ӧ���и������Լ�����Ķ����ԣ�������л��ϳ�ʱ����ʱ����Ҫ���Ƕ�ij�ֹ����Ž��б�������ʱ����Ҫ����ȡ������λ�á�
��.������ȡ����Ӧ�У����ϵ�ȡ������ȡ��λ�ò�����Ӱ�켴��λЧӦ��
(1)�ױ����Ȼ��������IJ������������£�
| ��λȡ������ | ��λȡ������ | ��λȡ������ |
�������У�25���¼ױ��Ȼ���Ӧ | 59.79% | 0.48% | 39.74% |
�����������мױ�������Ӧ | 63% | 3% | 34% |
�������е����ݣ��ɵó��Ľ�����
(2)�±��Dz�ͬ���������������Ӧ�����ڡ���λ����������
���� | C6H5-CH3 | C6H5-CH2CH3 | C6H5-CH(CH3)2 | C6H5-C(CH3)3 |
����� | 58.45% | 45.0% | 30.0% | 15.8% |
����� | 37.15% | 48.5% | 62.3% | 72.7% |
�Է�����AlCl3��������C6H5-CH3��Cl-C(CH3)3��Ӧ����Ҫ����ĽṹʽΪ ��
������
��.��������ȩ�ķ�Ӧ��ҩ����Ϻϳ��е���Ҫ��Ӧ֮һ������ȩ�뱥��NaHSO3��Һ���Է������·�Ӧ�����ø÷�Ӧ���Է���ȩ��ͪ�Ļ���
![]()
(1)��ҩ����Ϻϳ��г�����ȩ�ʹ���Ӧ������ȩ������ȩ�������෴Ӧ������½��С����磺 
�� ������ȩ��������Ӧ��Ҫ��֤��Ӧ��˳�����У��ɲ�ȡ�Ĵ�ʩ�� ��д��2������
��
����֪������Ԫ������Ԫ���ṹ����ȩ�Ƚ��ȶ���д�����Ҷ�����HOCH2CH2OH��������
��ȩ��ȩ���ķ�Ӧ����ʽ ��
(2)��д����ʹȩ��NaHSO3��Ӧ���ɵij��������ܽ���Լ��Ļ�ѧʽ ��д��2�֣����ڲ�ͬ�������ʡ��� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com