
���� �ٴ���Ϊ���ᣬ������ȫ���룬��pHʱ������Ũ�Ƚϴ�
�ڴ�����Ϊǿ�������Σ�ˮ��ʼ��ԣ�
��pH=7ʱ����Һ�����ԣ�pH��7ʱ����Һ�����ԣ�
�ܼ������������ƾ��壬c��CH3COO-���������ƴ���ĵ��룮
��� �⣺��1���ٴ���Ϊ���ᣬ������ȫ���룬��Ũ��������pH��С�����뷽��ʽΪCH3COOH?CH3COO-+H+����pHʱ������Ũ�Ƚϴ�����NaOH��Һ�࣬
�ʴ�Ϊ������CH3COOH?CH3COO-+H+������
�ڴ�����Ϊǿ�������Σ�ˮ��ʼ��ԣ�ˮ�ⷽ��ʽΪCH3COO-+H2O?CH3COOH+OH-���ʴ�Ϊ�����ԣ�CH3COO-+H2O?CH3COOH+OH-��
��pH=7ʱ����Һ�����ԣ�����c��Na+��=c��CH3COO-����c��H+��=c��OH-����pH��7ʱ����Һ�����ԣ���Һ���������ʵ�����Ũ�ȴ�С��ϵ����Ϊc��CH3COO-����c��Na+����c��H+����c��OH-�� ��c��CH3COO-����c��H+����c��Na+����c��OH-����
�ʴ�Ϊ��c��Na+��=c��CH3COO-����c��H+��=c��OH-����c��CH3COO-����c��Na+����c��H+����c��OH-�� ��c��CH3COO-����c��H+����c��Na+����c��OH-����
�ܼ������������ƾ��壬c��CH3COO-���������ƴ���ĵ��룬����ΪCH3COOH?CH3COO-+H+��������Ƶ��룬ʹc��CH3COO-��������ĵ���ƽ�������ƶ���c��H+����С��������ҺpH����
�ʴ�Ϊ��CH3COOH?CH3COO-+H+��������Ƶ��룬ʹc��CH3COO-��������ĵ���ƽ�������ƶ���c��H+����С��������ҺpH����
���� ���⿼���Ϊ�ۺϣ��漰������ʵĵ��룬���Ļ�ϼ����֪ʶ��������ѧ���ķ����Ŀ��飬Ϊ��Ƶ���㣬ע�����������ʵĵ����ص��Ӱ�����أ��ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ۢܢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ĵ��룺CH3COOH?CH3COO-+H+ | |
| B�� | ̼��������ˮ��Һ�еĵ��룺NaHCO3?Na++HCO3- | |
| C�� | �Ȼ�淋�ˮ�⣺NH4++H2O?NH4OH+H+ | |
| D�� | ̼��Ƶ��ܽ�ƽ�⣺CaCO3��s��?Ca2+��aq��+CO32-��aq�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | c��CH3COO-����c ��Na+�� | B�� | c��CH3COOH��+c��CH3COO-��=0.02mol/L | ||
| C�� | c��CH3COOH����c��CH3COO-�� | D�� | c��CH3COO-��+c��H+��=c ��Na+��+c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ۻ���������еĻ�ѧ�����ܺ������Ӽ� | |
| B�� | ���н���Ԫ�صĻ�����-�������ӻ����� | |
| C�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧ�����ȷ�Ӧ | |
| D�� | ��Ӧ�����Ȼ��Ƿ��ȱ��뿴�ɼ�����ʱ���յ��������¼�����ʱ�ͷŵ������Ĵ�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
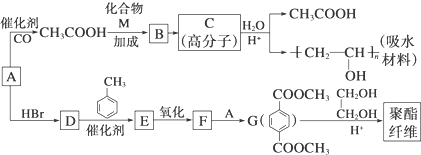
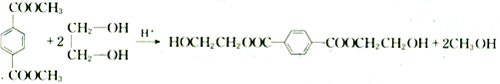
 �ϳ�
�ϳ� ������ͼ����ע����Ӧ��������
������ͼ����ע����Ӧ�������� $��_{����}^{����}$
$��_{����}^{����}$ $��_{��}^{NaOHˮ��Һ}$
$��_{��}^{NaOHˮ��Һ}$ $��_{��}^{����}$
$��_{��}^{����}$ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com