
Ϊ��ȷ��CH3COOH��H2CO3��H2SiO3������ǿ���������������ͼ��ʾװ�ã�һ��ʵ��ﵽĿ�ģ�������ѡ������������Һ����
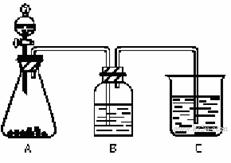 |
��1����ƿ��ʢ�������������е�ij�ֿ��������ι��壬�˹���Ϊ ����Һ©������ʢ�Լ��� ��
��2��װ��B����ʢ�Լ�Ϊ̼�����ƣ����Լ��������� ��
��3��װ��C�г��ֵ������� ��
��4�����б���(CH3CH2COOH)�ʹ���(CH3COOH)��ϵ (����ĸ)
�������(HCOOCH3)�ʹ���(CH3COOH)��ϵ (����ĸ)
A�� ͬλ��B��ͬ���칹C��ͬϵ��D��ͬ��������E��ͬһ����
��5����д��CH3COOH��CH3OH��������Ӧ
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�

��ע��Al(OH)3��Cu(OH)2��ʼ�ֽ���¶ȷֱ�Ϊ450���80�棩
��1����⾫����ʱ��������ӦʽΪ ������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ����ʽΪ ��
��2����������B�����Ϊ �������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ ��
(3)������չ�����һ����Ӧ�Ļ�ѧ����ʽ�� CuO + Al2O3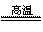 CuAlO2 + ��
CuAlO2 + ��
��4������ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0kg�����е�ͭ����ȫת��Ϊ mol CuAlO2��������Ҫ1.0mol��L-1��Al2(SO4)3��Һ L��
(5)CuSO4��ҺҲ�������Ʊ������������������ �����ˡ�ϴ�Ӻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���ǣ� ��
A. ճ����֬���Թܿ������ȵĴ�����Һϴ��
B. ʯ�͵ķ����ѻ����ѽⶼ�ǻ�ѧ�仯
C. ʳ���к������ᣬ��������Ҵ������õ�
D. ú����ˮ������Ӧ�Ƴ�ˮú����ˮú������Ҫ�ɷ�ΪCO��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ�NA ��ʾ�����ӵ�������ֵ���� ��
A�����³�ѹ�£�16g CH4���е���ԭ����Ϊ4NA
B��1mol�ǻ�����OH������10NA������
C��1mol��L-1 NaOH��Һ�к��е�������ΪNA
D����״���£�33. 6 L CH3CH2OH �к��еķ�����ĿΪ1. 5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ס������ֽ�����������Ƚϣ���˵���Ľ����Ա���ǿ���ǣ� ��
�� ��ˮ��Ӧ������ˮ��Ӧ����
�� �����ܴ��ҵ�����Һ���û���������
�� ������������ˮ������Ա��ҵ�����������ˮ�������ǿ
�� ��ij������Ӧʱ��ԭ�ӵõ�����Ŀ���ҵĶ�
A���٢� B���٢� C���٢ڢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧʽ��ֻ�ܱ�ʾһ�����ʵ��� �� ��
A.C3H7Cl B. C2H6O C. CH2Cl2 D.C2H4O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����еķ�Ӧ������ͬһ��Ӧ���͵��� (�� ��)
A���������ˮ���Ʊ������ɱ�ϩ��ˮ��Ӧ�Ʊ���
B���ɼױ������ƶ������ױ����ɼױ������Ʊ�����
C��������������ϩ���ɱ�ϩ���巴Ӧ��1,2�������
D����������Ҵ��������������ɱ���������ˮ���Ʊ�������Ҵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������װ��������ʵ��ɹ�ѡ��
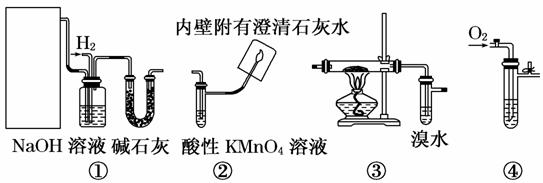
��.ʵ�����ü״����������õ���ȩ������֤��ȩ�����ʡ���ش��������⣺
(1)Ӧѡ���װ����___ _____(��д���)��
(2)����Ӳ�ʲ�������װ��ͭ�ۣ���д��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ__
_ __��
(3)ʵ������۲쵽��ˮ��ɫ��Ϊ������һ����ijѧϰС���ͬѧ������ֲ��룺
A. �����˼ӳɷ�Ӧ�� B. ������������Ӧ��
Ϊ̽�����ֲ�����ȷ��ѧϰС���ͬѧ��pH�Ʋ���ˮ��ɫǰ����Һ��pH�������Һ��pH�½�������Ϊ�������ַ�Ӧ����˵���������
________________ ________________________________________________________��
��.��ѡ��٢ۢ���֤��ϩ��������Ӧ�����ش��������⣺
(4)д��ʵ��������ϩ�Ļ�ѧ����ʽ_______________________________________��
(5)NaOH��Һ�������ǣ������㼴�ɣ�_____________________________________
_ _��
(6)ʵ������У����֢�����ˮ��ɫ�����û�ѧ����ʽ��ʾ��ˮ��ɫ��ԭ��_______________ _ ___��
(7)����ʲô�����˵����ϩ��������Ӧ����������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
200 mL 0.3 mol��L��1��K2SO4��Һ��100 mL 0.2 mol��L��1��Fe2(SO4)3��Һ��Ϻ�������Һ����ʱ����ı仯������Һ��SO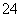 �����ʵ���Ũ��Ϊ
�����ʵ���Ũ��Ϊ
A��0.3 mol��L��1 B��0.4 mol��L��1 C��0.45 mol��L��1 D��0.5 mol��L��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com