
| |||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ijͬѧ��������ƽ�����ձ�����������ƽƽ����״̬����ͼ����ͼ�п��Կ�������ͬѧ�ڲ���ʱ��һ��������____________________���ձ���ʵ������Ϊ_______________g��
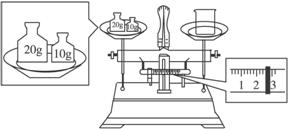
(2��ָ������ʵ���и����ڵ���������
A.������150 mL 0.2 mol��L-1NaCl��Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��

_______________________________________________________________________��
B.��ȥ�����е��Ȼ������壺
_______________________________________________________________________��
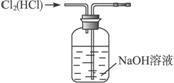
��3����98%�ܶ�Ϊ1.84 g��cm-3��Ũ��������1 000 mL 0.1 mol��L-1ϡ���ᡣ
��Ӧ����Ͳ��ȡŨ����___________________mL��
������������Һʱ��Ҫ�õ���������________________��������Ͳ�⣬д���������֣�
�����в��������������Һ���ʵ���Ũ��ƫ�͵���________________��
A.û�н�ϴ��Һת�Ƶ�����ƿ��
B.����ƿϴ����δ�����ﴦ��
C.����ʱ���ӹ۲�Һ��
D.ҡ�Ⱥ������۲죬������Һδ��̶��ߣ����õιܼӼ�������ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�������и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��10�֣�����Ϊ34 g��NH3�����ʵ����� mol���ڱ�״���µ����ԼΪ L ��0.1 mol H2S����Լ ����ԭ�ӣ���ͬ������NH3��H2S�з��Ӹ�����Ϊ ������100 mLŨ��Ϊ1 mol��L-1��ϡ���ᣬ��Ҫ��98%�ܶ�Ϊ1.84 g/cm3��Ũ���� mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣�����Ϊ34 g ��NH3�����ʵ����� mol���ڱ�״���µ����ԼΪ L ��0.1 mol H2S����Լ ����ԭ�ӣ���ͬ������NH3��H2S�з��Ӹ�����Ϊ ������100 mLŨ��Ϊ1 mol��L-1��ϡ���ᣬ��Ҫ��98%�ܶ�Ϊ1.84 g/cm3��Ũ���� mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�������и�һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com