
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣��ִ���п�ķ����ɷ�Ϊ��ʪ�������࣬������������п�ĸ���Ʒ�����ڸ߶��Խ������Իش������������.
��1������п�ǽ���п����Ҫ��ZnS��ͨ����ѡ������ʹ��ת��Ϊ����п���ٰ�����п�ͽ�̿��ϣ��ڹķ�¯�м�����1373-1573K��ʹп���������
��Ҫ��ӦΪ��2ZnS +3O2![]() 2ZnO+2SO2 ���ķ�¯�У�2C +O2
2ZnO+2SO2 ���ķ�¯�У�2C +O2![]() 2CO
2CO
�ķ�¯�У�ZnO+CO![]() Zn+ CO2
Zn+ CO2
�ӻ���п�����IJ����к����ֽ������ʼ�In2O3������������ȡ����ij�о�������Щ�о��������¡�ʵ�����漰����ȣ�ÿ����Һ�к�����������������Ľ���������ͼ1��������Һ������������������ͼ2��
�ٵ����Ϊ196ʱ�������ʵ���Ũ��Ϊ �� ��
�ڴӽ�Լԭ�Ϻͽ����ʿ��ǣ����˵���Ⱥ�Һ�̱ȷֱ�Ϊ�� �� �� �� ��
��2��ʪ����п����Ҫ��������Ϊ��
�������ȡ����Ҫ��Ӧ�����ӷ���ʽΪ�� �� ��
�ڴӱ��������ͳ������ԭ�ϽǶȣ���δ������������� �� ��
�۳�ȥ�����Һ�е���������H2O2�������ٵ���pHʹ֮�γ�Fe(OH)3������д��H2O2����Fe2+�����ӷ���ʽ �� ��
�������Һ������Cd2+,Ϊ�˷�ֹ����Ⱦ�������ӣ������������ʵIJ��죬��������������Һ���룬��֪Zn(OH)2����������һ��Ҳ�������ԣ���д����������ӷ���ʽ �� �� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ�����з�خ��2010��������Ĵ�ͳ�����ۻ�ѧ���� ���ͣ������
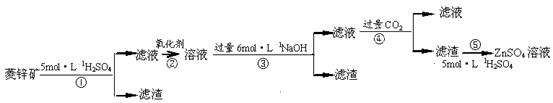
п������һ�ְ�ɫ���ϡ���ҵ������ZnSO4��BaS��Һ��϶��ɣ�BaS+ZnSO4 = ZnS��+BaSO4���������ǹ�ҵ�������̡���ش��й����⣺
��.ZnSO4��Һ���Ʊ����ᴿ
�й����ϣ���֪Zn(OH)2��Al(OH)3���ƣ������ڹ�����NaOH��Һ����Na2ZnO2��
��п�����Ҫ�ɷ���ZnCO3��������SiO2��FeCO3��Cu2(OH)2CO3�ȡ�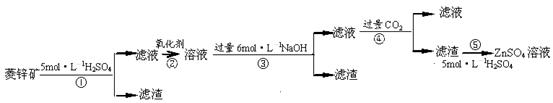 ��1������ʹ�õ���������������е� ������ţ��������� ��
��1������ʹ�õ���������������е� ������ţ��������� ��
| A��Cl2 | B��H2O2 | C��KMnO4 | D��ŨHNO3 |
 �й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ?mol-1
�й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ?mol-1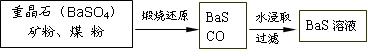
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ĸ���������¿���ѧ�Ծ� ���ͣ������
�ִ���п�ķ����ɷ�Ϊ��ʪ�������࣬������������п�ĸ���Ʒ�����ڸ߶��Խ������Իش�����������⣮
�Ż���п�ǽ���п����Ҫ��ZnS��ͨ����ѡ������ʹ��ת��Ϊ����п���ٰ�����п�ͽ�̿��ϣ��ڹķ�¯�м�����1373-1573K��ʹп�����������Ҫ��ӦΪ��
2ZnS +3O2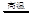 2ZnO+2SO2�� �ķ�¯�У�2C +O2
2ZnO+2SO2�� �ķ�¯�У�2C +O2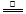 2CO
ZnO+CO
2CO
ZnO+CO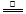 Zn
+ CO2
Zn
+ CO2
�ӻ���п�����IJ����к����ֽ������ʼ�In2O3������������ȡ����ij�о������Դ��о��������¡�ʵ�����漰����ȣ�ÿ����Һ�к�����������������Ľ���������ͼ1��������Һ������������������ͼ2
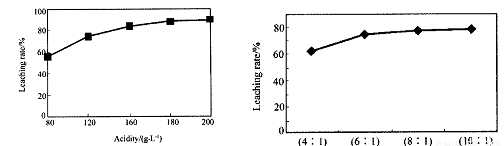
�ٵ����Ϊ196ʱ�������ʵ���Ũ��Ϊ ��
�ڴӽ�Լԭ�Ϻͽ����ʿ��ǣ����˵���Ⱥ�Һ�̱ȷֱ�Ϊ��_______��_______��
��ʪ����п����Ҫ��������Ϊ��
�ٴӱ��������ͳ������ԭ�ϽǶȣ���δ������������� ��
�ڳ�ȥ�����Һ�е���������H2O2�������ٵ���pHʹ֮�γ�Fe(OH)3������д��H2O2����Fe2+�����ӷ���ʽ ��
�������Һ������Cd2+��Ϊ�˷�ֹ����Ⱦ�������ӣ������������ʵIJ��죬��������������Һ���룬��֪Zn(OH)2����������һ��Ҳ�������ԣ���д����������ӷ���ʽ____________________��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
п������һ�ְ�ɫ���ϡ���ҵ������ZnSO4��BaS��Һ��϶��ɣ�BaS+ZnSO4 = ZnS��+BaSO4���������ǹ�ҵ�������̡���ش��й����⣺
��.ZnSO4��Һ���Ʊ����ᴿ
�й����ϣ���֪Zn(OH)2��Al(OH)3���ƣ������ڹ�����NaOH��Һ����Na2ZnO2��
��п�����Ҫ�ɷ���ZnCO3��������SiO2��FeCO3��Cu2(OH)2CO3�ȡ�
 ��1������ʹ�õ���������������е� ������ţ��������� ��
��1������ʹ�õ���������������е� ������ţ��������� ��
A.Cl2 B.H2O2 C.KMnO4 D.ŨHNO3
��2��д����Ӧ�ܵ����ӷ���ʽ�� ��
��3��Ϊ�˴ﵽ�ۺ����á����ܼ��ŵ�Ŀ�ģ����������в��� ������ �������ڲ�
�� �����в���ѡ��١��ڡ��ۡ��ܡ��ݣ���
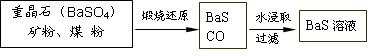
��.BaS��Һ���Ʊ�
![]() �й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ??mol-1
�й����ݣ� Ba��s����S��s����2O2��g����BaSO4��s������H = ��1473.2 kJ??mol-1
C��s���� ��O2��g����CO��g���� ��H = ��110.5 kJ??mol-1
Ba��s���� S��s����BaS��s���� ��H = ��460 kJ??mol-1
��4�����ջ�ԭ���Ȼ�ѧ����ʽΪ: ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com