

| ||
| ||
| ||
| ||
| FeBr3 |
| FeBr3 |
| ||
| ||
| ||
| ||
| ||
| FeBr3 |
| FeBr3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
I��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60����þ�ǴӺ�ˮ����ȡ�ġ���Ҫ�������£�
(1)Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ�� ��ҪʹMgSO4��ȫת��Ϊ
�����������Լ�����ӦΪ ��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ����� ��
(3)�Լ���ѡ�� ��д���䷴Ӧ�����ӷ���ʽ ��
(4)��ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ ��
���ڡ�����2�����ᵽ�ij����л����ش��������⣺
(1)���ڳ��³�ѹ�³� ̬��
(2)���³�ѹ����ֱ̬��������̼ԭ������ ��
(3)���ͼ����к�̼���ϸߵ��� ��
(4) д������Ũ���ᡢŨ������Һ���ȷ�Ӧ�Ļ�ѧ����ʽ���ýṹ��ʽ��д�� ��
(5) д���Ҵ��������������Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
I��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60����þ�ǴӺ�ˮ����ȡ�ġ���Ҫ�������£�
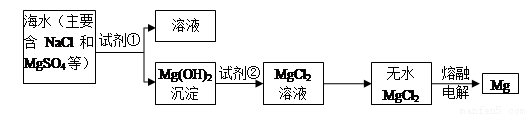
(1)Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ�� ��ҪʹMgSO4��ȫת��Ϊ
�����������Լ�����ӦΪ ��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ����� ��
(3)�Լ���ѡ�� ��д���䷴Ӧ�����ӷ���ʽ ��
(4)��ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ ��
���ڡ�����2�����ᵽ�ij����л����ش��������⣺
(1)���ڳ��³�ѹ�³� ̬��
(2)���³�ѹ����ֱ̬��������̼ԭ������ ��
(3)���ͼ����к�̼���ϸߵ��� ��
(4) д������Ũ���ᡢŨ������Һ���ȷ�Ӧ�Ļ�ѧ����ʽ���ýṹ��ʽ��д�� ��
(5) д���Ҵ��������������Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�걱���г�������һ��ѧ����ĩ���Ի�ѧ�� ���ͣ������
(10��)��ˮ�л�ѧ��Դ���ۺϿ����������ܵ������ĸ߶����ӡ�Br2��Mg�����ֵ��ʶ����ԴӺ�ˮ����ȡ����ͼΪ��ȡ���ǵIJ��裺

��ش�
I���Ӻ�ˮ����ȡ����ռ�����������1/3����Ҫ�����������������еĿ�����������
��1����ȡBr2ʱ����һ��ͨ��Cl2������Ӧ�����ӷ���ʽ��__________________��
��2���������з�Ӧ�����ӷ���ʽ��________________________________________��
�ɣ�1������2����֪��SO2��Cl2��Br2����������������ǿ������˳����
__________________���� �á�����ʾ��
II��þ����Ͻ�����;�ܹ㷺�Ľ������ϣ���Ŀǰ������60%��þ���ǴӺ�ˮ�а�����������ȡ�ġ�
��1������������Ϊ��ʹMgSO4��ȫת��ΪMg(OH)2���Լ��ٿ���ѡ��___________��д��ѧʽ����
��2�������Լ��ں�Ӧ�����ӷ���ʽ��_________________________________��
��3������ٰ������ȡ���������ȴ���ᾧ��_________��
��4��ͨ��ʱ��ˮMgCl2������״̬�·�Ӧ�Ļ�ѧ����ʽ��
___________________________________________________��
III�������������������þ��ͬʱ�������Ƶ������������ʣ������Ʊ��ͻ��������þ�����ᡣ���������ǣ�
�ٽ��Ȼ�þ���壨MgCl2��6H2O�����ȵ�523OC���ϣ��þ�����Էֽ�õ��ͻ��������þ��������̬���������һ�����峣����Ϊ��ɫҺ�塣
�ڽ�����������ȴ�����£��ٸ�����Ҫ�����벻ͬ����ˮ���Ϳɵõ���ͬŨ�ȵ����ᡣ
��1��MgCl2��6H2O��523OC���Ϸֽ�Ļ�ѧ����ʽ��_____________________________��
��2������1mol MgCl2��6H2O�ֽ����õķǹ����������ȡ�ܶ�Ϊ1.19g/cm3��������Һ168mL�����ˮ__________g����ȷ��0.1���������������ʵ����ʵ���Ũ����____________mol/L����ȷ��0.1����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60����þ�ǴӺ�ˮ����ȡ�ġ���Ҫ�������£�
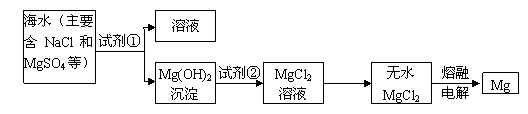
(1)Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ�������� ��ҪʹMgSO4��ȫת��Ϊ
�� �����������Լ�����ӦΪ������������ ��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ����������������� ��
(3)�Լ���ѡ���������� ��д���䷴Ӧ�����ӷ���ʽ���������������������������� ��
(4)��ˮMgCl2������״̬�£�ͨ������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ�������� ��
II�����ڡ�����2�����ᵽ�ij����л����ش��������⣺
(1)���ڳ��³�ѹ�³����� ̬��
(2)���³�ѹ����ֱ̬��������̼ԭ�������������� ��
(3)���ͼ����к�̼���ϸߵ����������� ��
(4) д������Ũ���ᡢŨ������Һ���ȷ�Ӧ�Ļ�ѧ����ʽ���ýṹ��ʽ��д���������������� ��
(5) д���Ҵ��������������Ӧ����ʽ �������������� ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com