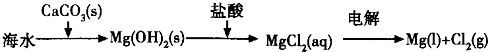
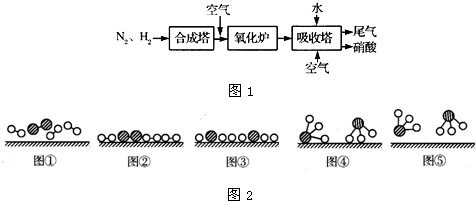
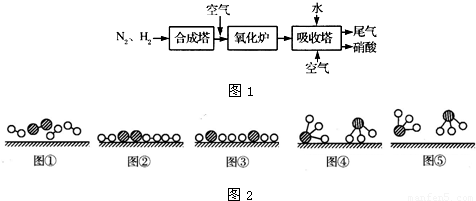
��ͼ1��ʾ�ǹ�ҵ������������̣�

�ϳ�������������ý������¯������Pt-Rh�Ͻ�������ش��������⣺
��1��1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰���2007�껯ѧ�Ҹ����?���ض��ڹ����о���֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ����ͼ2��ʾ��

��

��

�ֱ��ʾN
2��H
2��NH
3��ͼ�ݱ�ʾ���ɵ�NH
3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���
ͼ�ڱ�ʾN2��H2�������ڴ������棬ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ������
ͼ�ڱ�ʾN2��H2�������ڴ������棬ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ������
��
��2���ϳɰ���Ӧ�Ļ�ѧ����ʽΪN
2��g��+3H
2��g��?2NH
3��g����д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=
����һ���¶Ⱥ�ѹǿ�£���H
2 ��N
2 ��3��1�����֮�ȣ���Ϻ����ϳ�������Ӧ�ﵽƽ��ʱ��ƽ��������NH
3 ���������Ϊ15%����ʱH
2 ��ת����Ϊ
26%
26%
��
��3����֪��4NH
3��g��+3O
2��g��=2N
2��g��+6H
2O��g����H=-1 266.8kJ/mol
N
2��g��+O
2��g��=2NO��g����H=+1 80.5kJ/mol�������������Ȼ�ѧ����ʽΪ
4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol
4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol
��
��4����������ͨ�������Ŀ����
ʹNOѭ�����ã�ȫ��ת��������
ʹNOѭ�����ã�ȫ��ת��������
��


 ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���

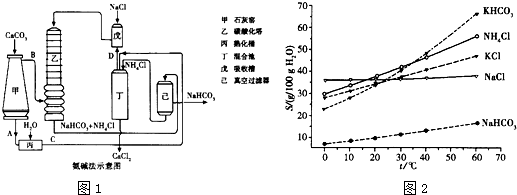
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
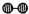 ��
��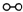 ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��
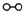

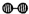 ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��