
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
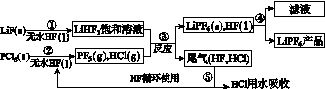
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
(1)�ڢٲ���Ӧ����ˮHF��������________________��________________����Ӧ�豸�����ò������ʵ�ԭ����______________________________________________(�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%��________��Һ��ϴ��
(2)������������ˮ�����½��У��ڢ۲���Ӧ��PF5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ��____________________________________��
(3)�ڢܲ�������õķ�����________���ڢݲ�����β����HF��HCl���õķ�����________��
(4)LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒw g�����Li�����ʵ���Ϊn mol�������Ʒ��LiPF6�����ʵ���Ϊ________mol(�ú�w��n�Ĵ���ʽ��ʾ)��
(1)��Ӧ��ܼ�
SiO2��4HFSiF4����2H2O��NaHCO3
(2)PF5��4H2OH3PO4��5HF
(3)���ˡ�����
(4)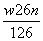
[����] (1)����֪��֪����ʱHFΪҺ̬����Ӧ�ٵõ�LiHF2������Һ���ɼ��ڷ�Ӧ������ˮHF������������Ӧ����ܼ�����������Ҫ�ɷ�ΪSiO2��SiO2����HF��Ӧ����SiF4�����H2O�����Է�Ӧ�豸�����ò������ģ�HF����ˮ�õ�����ᣬ���ϴ��ʱ��ѡ�ü��Ժ���������Һ��ϴ�ӣ���2%��NaHCO3��Һ��(2)F�����ᣬ���PF5ˮ��õ���������һ��Ϊ���������ᡣ(3)�ڢܷ����õ���Һ�����ƷLiPF6���ɼ����뷽���ǹ��ˣ���HCl��HF�ķе��֪����HCl��HF�Ļ���ֻ�轫������彵������(HFת��ΪҺ̬)�ɷ��롣(4)��LiPF6��LiF�Ļ�ѧʽ�����á���غ㡱֪w g LiPF6��LiF���������ʵ���Ϊn mol����w g��Ʒ��LiPF6��LiF�����ʵ����ֱ�Ϊx mol��y mol��������ã�x��y��n��152x��26y��w����ʽ�������x��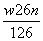 ��
��


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijǿ������ҺX����Ba2����Al3����NH ��Fe2����Fe3����CO
��Fe2����Fe3����CO ��SO
��SO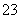 ��SO
��SO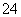 ��
��
Cl����NO �е�һ�ֻ��֣�ȡ����Һ����ʵ�飬ʵ���������£�
�е�һ�ֻ��֣�ȡ����Һ����ʵ�飬ʵ���������£�
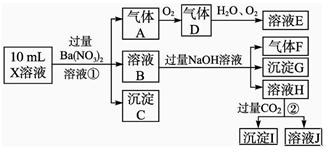
����������Ϣ���ش��������⣺
(1)��ҺX�г�H����϶����е�������_____________________________________��
(2)д���й����ӷ���ʽ��
�����������A__________________;��������ɳ���I__________________��
(4)����ⶨA��F��I��Ϊ0.01 mol,10 mL X��Һ��n(H��)��0.04 mol��������C���ʵ���0.07 mol����˵������Һ����ȷ���������Ӵ��ڵ�������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ߡ����ʹ�õ�ȼ������ƫ�����£���ṹ���Կ�������������һ����������NH2������˰��������е�������ԭ�ӣ����й���ƫ�����µ�˵������ȷ����
A����̼���⡢������Ԫ����� B������N��Cԭ�Ӷ���ͬһƽ����
C�����������е�Nԭ�Ӿ���sp3�ӻ� D������ʽΪC2H8N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NaOH��Һ�ζ�����ʵ���У������õ�����(����)
A����̪ B��Բ����ƿ
C����ƿ D����ʽ�ζ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼʾʵ����ȷ����(����)
 ��
��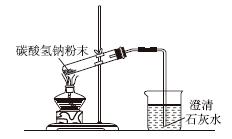
A.��ȥ������Һ,�еIJ���� B��̼���������ȷֽ�
��
����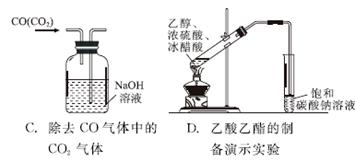
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
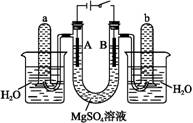
����������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
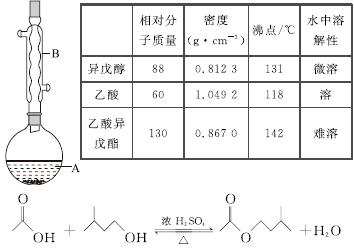
ʵ�鲽�裺
��A�м���4.4 g���촼��6.0 g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50 min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143 ����֣�������������3.9 g��
�ش��������⣺
(1)����B��������________________��
(2)��ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����____________________________________���ڶ���ˮϴ����ҪĿ����________________��
(3)��ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��________(����)��
a��ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b��ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
(4)��ʵ���м�����������Ŀ����___________________________________________��
(5)ʵ���м���������ˮMgSO4��Ŀ����________��
(6)����������У�����ѡ��װ����ȷ����________(����)��
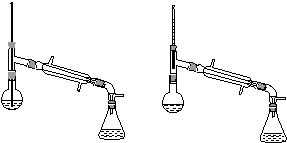
������a��������������������������b.
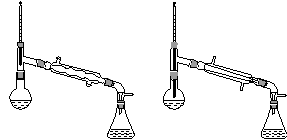
������c��������������������������d.
(7)��ʵ��IJ�����________(����)��
a��30% ��b��40% c��60% d��90%
(8)�ڽ����������ʱ������130 ��㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ__________(��ߡ��͡�)����ԭ����______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����װ��Ӧ����ʵ�����������������Ȼ��̵�ʵ�飬�ܴﵽʵ��Ŀ�ĵ���(����)
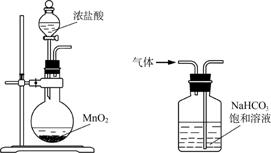
�����������������ס�������������������
����������������������������������
A����װ�ü���ȡ����
B����װ���ҳ�ȥ�����е������Ȼ���
C����װ�ñ�����������̺��Ȼ�����Һ
D����װ�ö������Ȼ�����Һ��MnCl2��4H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
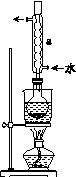
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)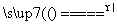 Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O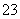 ��I2===S4O
��I2===S4O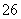 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
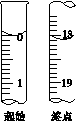
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO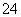 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
��1����ҵұ�����Ļ�ѧ����ʽ������������������������
��2����ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��
������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ��
�ٸõ��۵�������Ӧʽ������������������������
��ͨ�翪ʼ������������ҺpH�����������ԭ��������������������������
�۳�ȥ���ʺ������������Һ����Һ������������(��д��A����B��)������
��3�����ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)+10C(s) 6CaO(s)+P4(s)+10CO(g)����H1=+3 359��26 kJ��mol-1
6CaO(s)+P4(s)+10CO(g)����H1=+3 359��26 kJ��mol-1
CaO(s)+SiO2(s) CaSiO3(s)����H2=-89��61 kJ��mol-1
CaSiO3(s)����H2=-89��61 kJ��mol-1
2Ca3(PO4)2(s)+6SiO2(s)+10C(s) 6CaSiO3(s)+P4(s)+10CO(g)����H3
6CaSiO3(s)+P4(s)+10CO(g)����H3
��H3=�������� kJ��mol-1��
 ��4�����ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
��4�����ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
2SO2(g)+O2(g) 2SO3(g)����H1=-197 kJ��mol-1��
2SO3(g)����H1=-197 kJ��mol-1��
H2O(g) H2O(l)����H2=-44 kJ��mol-1��
H2O(l)����H2=-44 kJ��mol-1��
2SO2(g)+O2(g)+2H2O (g) 2H2SO4(l)����H3=-545 kJ��mol-1��
2H2SO4(l)����H3=-545 kJ��mol-1��
��SO3(g)��H2O(l)��Ӧ���Ȼ�ѧ����ʽ��������������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com