
(1)�á�84����Һ�������ʵ���Ũ��ԼΪ________mol��L��1��
84����Һ
��Ч�ɷ� NaClO
��� 1 000 mL
�������� 25%
�ܶ� 1.19 g��cm��3
(2)ijͬѧȡ100mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na��)��______mol��L��1��
(3)��ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480mL��NaClO��������Ϊ25%������Һ������˵����ȷ����________(����ĸ)��
A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɺ����������Һ����
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
D����Ҫ����NaClO���������Ϊ143.0g
(4)��84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84g��cm��3)��Ũ��������2L 2.3mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________mol��L��1��
������Ũ��������Ϊ________mL��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ɹŸ����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��Ʒ������Ϊ�������������������ܼ�����֪��Ʒ���Ľṹ��ʽ��ͼ��ʾ,������˵���������( )
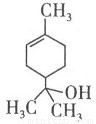
A��1 mol����������ܺ�1 mol���������ӳɷ�Ӧ
B�������к��еĹ�����Ϊ�ǻ���̼̼˫��
C���������ܺ����ᷢ��������Ӧ
D�������ʵķ���ʽΪC9H18O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��100 mol FeBr2����Һ�У�ͨ��3 mol Cl2 ����Ӧ����Һ��Br-��Cl-�����ʵ���Ũ����ȣ�����Һ�з�����Ӧ�����ӷ���ʽΪ ( )
A��2Fe2++Cl2 = 2Fe3++2Cl- B��2Br-+Cl2 = Br2+2Cl-
C��4Fe2++2Br-+3Cl2 = Br2+4Fe3++6Cl- D��2Fe2++4Br-+3Cl2 = 2Br2+2Fe3++6Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��Na��Mg��Al��Zn���ֽ����е�������ɵĻ����30g,��������ϡ���ᷴӦ����1g�������������бض��еĽ����ǣ� ��
A��Na B��Mg C��Al D��Zn
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NAΪ�����ӵ���������������������ȷ���ǣ� ��
A���ڱ�״���£�22.4L��C6H14����������ΪNA
B����ͬ������H2��Cl2��ȣ�H2�ķ�������
C��2.3g����Na���Na+ʱʧȥ�ĵ�����ĿΪ0.1NA
D����1L0.1mol/L��HAc��Һ�У�����Ac����HAc֮��Ϊ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ������ٽ��Կ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪������Br2��Fe3+��FeBr2��Һ��ͨ��һ������Cl2��������Ӧ�����ӷ���ʽΪ��a Fe2++b Br-+c Cl2��d Fe3++ e Br2+ f Cl-,����ѡ���е����������ӷ���ʽ�е�a��b��c��d��e��fһһ��Ӧ�����в����Ϸ�Ӧʵ�ʵ��� ( )
A��2 4 3 2 2 6 B��0 2 1 0 1 2
C��2 0 1 2 0 2 D��2 2 2 2 1 4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ������ٽ��Կ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ڻ�ѧѧϰ��ʹ������ı�ʾ��������ֱ�ۡ������Ч��,���б����в���ȷ����( )
A����������Һ��pH������ԵĹ�ϵ:
B����Ļ������л��ϼ����������ԡ���ԭ�ԵĹ�ϵ:
C��Fe��Cl2�е�ȼ�ղ���:
D����ˮ��SO2��Ӧ����Һ�е����:
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���и������ʣ�ǰ�������ʱ������ǰ���Ƿ������������ͬһ���ӷ���ʽ��ʾ���ǣ���ԭ�ԣ�Fe2+��Br����C1����
A��ϡ���ᣬ̼������Һ B��ϡ���ᣬ̼��������Һ
C��̼��������Һ������ʯ��ˮ D����ˮ���廯������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꼪��ʡ�߶���9���¿���ѧ���������棩 ���ͣ�ѡ����
ij�¶��£�Ag2SO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ����
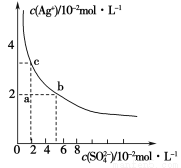
A��a���ʾAg2SO4�IJ�������Һ������Na2SO4���岻��ʹ��Һ��a��䵽b��
B��a���ʾAg2SO4�IJ�������Һ����������ʹ��Һ��a��䵽c��
C��0.04 mol��L��1��AgNO3��Һ��0.2 mol��L��1��Na2SO4��Һ����������Ag2SO4��������
D��Ag2SO4���ܶȻ�����(Ksp)Ϊ1��10��3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com