
| ��ѧ�� | Si-O | Si-Cl | H-H | H-Cl | Si-Si | Si-C |
| ����/kJ?mol-1 | 460 | 360 | 436 | 431 | 176 | 347 |
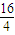 =28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ��ݴ˽����⣮
=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ��ݴ˽����⣮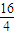 =28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ�
=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����M��E��=2M��O��=2×16=32������EΪSԪ�أ�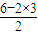 =3��û�й¶Ե��ӣ���Ϊƽ�������Σ�
=3��û�й¶Ե��ӣ���Ϊƽ�������Σ� ����1mol�������Si-Si��Ϊ1mol×4×
����1mol�������Si-Si��Ϊ1mol×4× =2mol����ӦSiCl4��g��+2H2��g��=Si��s��+4HCl��g���ķ�Ӧ�ȡ�H=4×360kJ/mol+2×436kJ/mol-2×176kJ/mol-4×431kJ/mol=236kJ/mol����Ϊ���ȷ�Ӧ��
=2mol����ӦSiCl4��g��+2H2��g��=Si��s��+4HCl��g���ķ�Ӧ�ȡ�H=4×360kJ/mol+2×436kJ/mol-2×176kJ/mol-4×431kJ/mol=236kJ/mol����Ϊ���ȷ�Ӧ��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�






�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
32 16 |
32 16 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�






�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com