
���б����еĸ�����������������ͼ���ʾ����
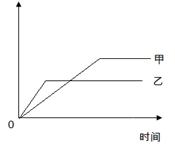
| ��Ӧ | ������ | �� | �� | |
| A�� | �¶���ͬ�����һ������ͬ�������У� N2 +3H2 | NH3�����ʵ��� | 1mol N2 3mol H2 | 1mol N2 1mol H2 |
| B�� | �������ļء��Ʒֱ���������ˮ��Ӧ | H2������ | �� | �� |
| C�� | ��ͬ�¶��£������1��1�� H2��I2��Ӧ H2+I2 | HI��Ũ�� | 10������ѹ | 20������ѹ |
| D�� | ��ͬ������ SO3������ͬ�����У� 2SO3 | SO3��ת���� | 500�� | 400�� |
 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
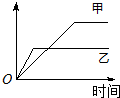 ��2009?Ϋ��һģ�����б����еĸ�������������������ͼ�����߱�ʾ���ǣ������� ��2009?Ϋ��һģ�����б����еĸ�������������������ͼ�����߱�ʾ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б����еĸ�������������������Ӧѡ���е�ͼ�����߱�ʾ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ��ˮ��ѧ�����ڶ���ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ���ѡ��
���б����еĸ����������������ͼ�����߱�ʾ���� �� �� 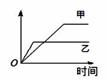
| | ������ Ӧ | ������ | �� | �� |
| A | ��ͬ�����İ�����ͬһ������ 2NH3  ��N2 + 3H2 ��N2 + 3H2 | ������ת���� | 500�� | 400�� |
| B | �������ء��Ʒֱ�������ˮ��Ӧ | H2���� | �� | �� |
| C | ������ɱ�ĺ�ѹ�����У������ 1�U3��N2��H2�� N2 + 3H2 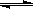 ��2NH3 ��2NH3 | ������Ũ�� | ���ԸߵĴ��� | ����һ��Ĵ��� |
| D | 2 molSO2��1 molO2������ͬ�¶���2SO2 + O2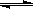 ��2SO3 ��2SO3 | SO3���ʵ��� | 2������ѹ | 10������ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�갲��ʡ��У�о������������1�£����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���б����еĸ�������������������ͼ�����߱�ʾ����

????????????? ��Ӧ????????????? ������????????????? ��????????????? ��
A????????????? �������ء��Ʒֱ�������ˮ��Ӧ????????????? H 2����?????????????
��?????????????
��
B????????????? ��ͬ������������ͬһ������
2NH3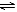 N2+3H2???? ��H >0????????????? ������ת����????????????? 500��????????????? 400��
N2+3H2???? ��H >0????????????? ������ת����????????????? 500��????????????? 400��
C????????????? ������ɱ�ĺ�ѹ�����У������1:3��N2��H2��N2+3H2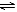 2NH3????????????? ������Ũ��????????????? ����һ��Ĵ���????????????? ���ԸߵĴ���
2NH3????????????? ������Ũ��????????????? ����һ��Ĵ���????????????? ���ԸߵĴ���
D????????????? 2molSO2��lmolO2������ͬ�¶���2SO2(g)+O2(g)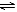 2SO3(g)????????????? SO3���ʵ���????????????? 2������ѹ????????????? 10������ѹ
2SO3(g)????????????? SO3���ʵ���????????????? 2������ѹ????????????? 10������ѹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ���˰�У�߶���ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
���б����еĸ�������������������Ӧѡ���е�ͼ�����߱�ʾ����
|
ѡ�� |
��Ӧ |
������ |
�� |
�� |
|
A |
���Ρ���С����Ľ�����ˮ��Ӧ |
��Ӧ���� |
Mg] |
Na |
|
B |
4 mL 0.01 mol/L��KMnO4��Һ���ֱ�Ͳ�ͬŨ�ȵ�H2C2O4(����)��Һ��2mL��Ӧ |
0.1 mol/L��H2C2O4��Һ |
0.2 mol/L��H2C2O4��Һ |
|
|
C |
5 mL 0.1 mol/L Na2S2O3��Һ��5 mL 0.1 mol/L H2SO4��Һ��Ӧ |
��ˮ |
��ˮ |
|
|
D |
5 mL 4%�Ĺ���������Һ�ֽ�ų�O2 |
��MnO2��ĩ |
��MnO2��ĩ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com