
�Կ��淴ӦaA��g����bB��g��![]() cC��g����dD��g���ﵽƽ��ʱ�����ʵ�
Ũ�����㣺
cC��g����dD��g���ﵽƽ��ʱ�����ʵ�
Ũ�����㣺![]() ��KΪһ�������䷴Ӧ��Kֵֻ���¶��йأ���һ��ӦCO��g����H2O��g��=CO2��g����H2��g������H��0����850��ʱ��K��1��
��KΪһ�������䷴Ӧ��Kֵֻ���¶��йأ���һ��ӦCO��g����H2O��g��=CO2��g����H2��g������H��0����850��ʱ��K��1��
��1�������µ�950�棬�ﵽƽ��ʱ��K________1�����������
��2��850��ʱ���̶��ݻ��ܱ������У����������ʼŨ��c(CO)��0.01mol��L��1�� c��H2O����0.05mol��L��1��c��CO2����0.01mol��L��1��c��H2����0.01mol��L��1���� Ӧ��ʼʱH2O���������ʱ���������________�����С������ȷ������
��3�������¶ȡ�������������䣬���������г���������H2S���壬�����´ﵽƽ�� ʱ��CO���������ʱȳ�H2S֮ǰ________��������С������ȷ������������________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��1�������µ�950�棬�ﵽƽ��ʱ��K________1�����������
��2��850��ʱ���̶��ݻ��ܱ������У����������ʼŨ��c(CO)��0.01mol��L��1�� c��H2O����0.05mol��L��1��c��CO2����0.01mol��L��1��c��H2����0.01mol��L��1���� Ӧ��ʼʱH2O���������ʱ���������________�����С������ȷ������
��3�������¶ȡ�������������䣬���������г���������H2S���壬�����´ﵽƽ�� ʱ��CO���������ʱȳ�H2S֮ǰ________��������С������ȷ������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
�Կ��淴ӦaA��g����bB��g��![]() cC��g����dD��g���ﵽƽ��ʱ�������ʵ����ʵ���Ũ���������¹�ϵ��
cC��g����dD��g���ﵽƽ��ʱ�������ʵ����ʵ���Ũ���������¹�ϵ��
 ��K��Ϊһ��������K��Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��� �ء����з�Ӧ��
��K��Ϊһ��������K��Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��� �ء����з�Ӧ��
CO��g����H2O��g����CO2��g����H2��g����H<0����850��ʱ��K��1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK________1������ڡ���С�ڡ����ڡ�����
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0molCO��3.0molH2O1.0molCO2 ��xmolH2���� �ٵ�x��5.0ʱ������ƽ����________���������Ӧ�����淴Ӧ���������ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
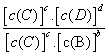 ��K��Ϊһ��������K��Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��� �ء����з�Ӧ��
��K��Ϊһ��������K��Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��� �ء����з�Ӧ��
CO��g����H2O��g����CO2��g����H2��g����H<0����850��ʱ��K��1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK________1������ڡ���С�ڡ����ڡ�����
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0molCO��3.0molH2O1.0molCO2 ��xmolH2���� �ٵ�x��5.0ʱ������ƽ����________���������Ӧ�����淴Ӧ���������ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��8�֣��Կ��淴ӦaA(g)��bB(g) cC(g)��d��(g)�ﵽƽ��ʱ�������ʵ����ʵ���Ũ���������¹�ϵ��
cC(g)��d��(g)�ﵽƽ��ʱ�������ʵ����ʵ���Ũ���������¹�ϵ�� (Ϊһ����)��K��Ϊ��ѧƽ�ⳣ��������ֵֻ���¶��йء����з�Ӧ��CO(g)��H2O(g)
(Ϊһ����)��K��Ϊ��ѧƽ�ⳣ��������ֵֻ���¶��йء����з�Ӧ��CO(g)��H2O(g) CO2(g)��H2(g)����H<0����850��ʱ��K��1��
CO2(g)��H2(g)����H<0����850��ʱ��K��1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK 1(����ڡ�����С�ڡ����ڡ�)��
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0 mol CO��3.0 mol H2O��1.0 mol CO2��x mol H2���ٵ�x��5.0ʱ������ƽ���� (�����Ӧ�����淴Ӧ��)�����ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ����������� ��
��3����850��ʱ������x��5.0��x��6��0���������ʵ�Ͷ�ϲ��䣬��������Ӧ�ﵽƽ����H2����������ֱ�Ϊa����b������a b(����ڡ�����С�ڡ����ڡ�)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com