

������������⣺
��1������������˳����������ӣ�����ӿڵı�ţ�Ϊ_______________��
��2����Ӧǰ��ͨ��N2��Ŀ����__________________________________________��
��3��ȷ�������к�NO��������__________________________________________��
��4��װ��F��������_________________________________________________��
��5�����O2������װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ɽ��ʡΫ��������2011��2012ѧ���һ��ѧ��ģ��ѧ���϶���⻯ѧ���� ���ͣ�058
ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ����ش��������⣺
(1)����ʵ��������������________(��д���)��
a����ֽ�����Թ���װ��ĩ״ҩƷʱ���Թ�Ӧ�Ⱥ����ֱ��
b����ȡ�������ƹ���ʱ��Ӧ���������ƹ���ֱ�ӷ�����������ڣ��ұ����̷�����
c���Թܡ��ձ�����Ͳ������ƿ�������þƾ���ֱ�Ӽ���
d������ֽ������������ʱ����������ֽ��ˮ��ϴ������۲���ֽ��ɫ�ı仯
e����������ζʱ��Ӧȡһƿʢ������ļ���ƿ���Դ���Ƭ�����������ȵ���ƿ���ȶ���ʹ������������Ʈ���ǿף�����ζ
(2)ʵ������Ҫ450 mL��0.1 mol��L��1��NaOH��Һ��500 mL��0.5 mol��L��1������Һ����ش���
�����⣺
������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����________(�����)����ͼ�����������⣬����������Һ����Ҫ�IJ���������________��

��������ƿ��ʹ�÷����У����в�����ȷ����________(��д���)��
a��ʹ������ƿǰ�����Ƿ�©ˮ
b������ƿ��ˮϴ�������ô�����Һϴ��
c��������Һʱ����������Һ�壬����Ͳȡ�����ò���������ע������ƿ�У�Ȼ�������ˮ��
d���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ�
������450 mL��0.1 mol��L��1��NaOH��Һ��ʵ�鲽�����£�
a������Ӧ��ȡ�������ƹ��������Ϊ________g��
b�������������ƹ��壮
c�����ձ��е���Һע������ƿ��������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲע������ƿ��
d��������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e���Ǻ�ƿ�����������µߵ���ҡ�ȣ�
f������������ƿ�м�����ˮ���̶�����1��2 cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�����������ȷ����˳��Ϊ________(����ű�ʾ)��
�ܸ��ݼ����֪��������Ͳ��ȡ��������Ϊ98�����ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ________mL�����ʵ������15 mL��20 mL��50 mL��Ͳ��Ӧѡ��________mL��Ͳ��ã�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ��һ12��ѧ����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��16�֣�ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ����ش��������⣺
��1������ʵ��������������____________________����д��ţ���
a. ��ֽ�����Թ���װ��ĩ״ҩƷʱ���Թ�Ӧ�Ⱥ����ֱ��
b. ��ȡ�������ƹ���ʱ��Ӧ���������ƹ���ֱ�ӷ�����������ڣ��ұ����̷�����
c. �Թܡ��ձ�����Ͳ������ƿ�������þƾ���ֱ�Ӽ���
d.����ֽ������������ʱ����������ֽ��ˮ��ϴ������۲���ֽ��ɫ�ı仯
e.��������ζʱ��Ӧȡһƿʢ������ļ���ƿ���Դ���Ƭ�����������ȵ���ƿ���ȶ���ʹ������������Ʈ���ǿף�����ζ
��2��ʵ������Ҫ450mL 0.1mol��L-1NaOH��Һ��500mL 0.5 mol��L-1������Һ����ش���
������:
������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ��������� ��

��������ƿ��ʹ�÷����У����в�����ȷ����____________����д��ţ���
a��ʹ������ƿǰ�����Ƿ�©ˮ
b������ƿ��ˮϴ�������ô�����Һϴ��
c��������Һʱ����������Һ�壬����Ͳȡ�����ò���������ע������ƿ�У�Ȼ�������ˮ��
d���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
������450 mL 0.1 mol��L-1 NaOH��Һ��ʵ�鲽�����£�
a.����Ӧ��ȡ�������ƹ��������Ϊ________g��
b.�����������ƹ��塣
c.���ձ��е���Һע������ƿ��������������ˮϴ���ձ��ڱ�2~3�Σ�ϴ��ҺҲע����
��ƿ��
d.������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e.�Ǻ�ƿ�����������µߵ���ҡ�ȡ�
f.����������ƿ�м�����ˮ���̶�����1~2cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����
�̶������С�
�����������ȷ����˳��Ϊ_______________________������ű�ʾ����
�ܸ��ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ
mL�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �£���A��B��C��D��E��ƿ�зֱ��������ϸ��˿������ʳ��ˮ��ϸ��˿��������ˮ��ϸ��˿����ʳ��ˮ��ȫ��û��ϸ��˿������ˮ��ȫ��û��ϸ��˿��Ȼ����װ������ͼ��ʾ������װ�ã�ÿ��һ��ʱ�����������ˮ�������ĸ߶ȡ�������±���ʾ��������������Ϊ����ˮ�������ĸ߶�/mm����
�£���A��B��C��D��E��ƿ�зֱ��������ϸ��˿������ʳ��ˮ��ϸ��˿��������ˮ��ϸ��˿����ʳ��ˮ��ȫ��û��ϸ��˿������ˮ��ȫ��û��ϸ��˿��Ȼ����װ������ͼ��ʾ������װ�ã�ÿ��һ��ʱ�����������ˮ�������ĸ߶ȡ�������±���ʾ��������������Ϊ����ˮ�������ĸ߶�/mm����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ����ش��������⣺
��1������ʵ��������������____________________����д��ţ���
a. ��ֽ�����Թ���װ��ĩ״ҩƷʱ���Թ�Ӧ�Ⱥ����ֱ��
b. ��ȡ�������ƹ���ʱ��Ӧ���������ƹ���ֱ�ӷ�����������ڣ��ұ����̷�����
c. �Թܡ��ձ�����Ͳ������ƿ�������þƾ���ֱ�Ӽ���
d.����ֽ������������ʱ����������ֽ��ˮ��ϴ������۲���ֽ��ɫ�ı仯
e.��������ζʱ��Ӧȡһƿʢ������ļ���ƿ���Դ���Ƭ�����������ȵ���ƿ���ȶ���ʹ������������Ʈ���ǿף�����ζ
��2��ʵ������Ҫ450mL 0.1mol��L-1NaOH��Һ��500mL 0.5 mol��L-1������Һ����ش���
������:
������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ��������� ��
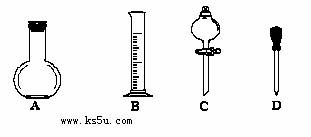
��������ƿ��ʹ�÷����У����в�����ȷ����____________����д��ţ���
a��ʹ������ƿǰ�����Ƿ�©ˮ
b������ƿ��ˮϴ�������ô�����Һϴ��
c��������Һʱ����������Һ�壬����Ͳȡ�����ò���������ע������ƿ�У�Ȼ�������ˮ��
d���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
������450 mL 0.1 mol��L-1 NaOH��Һ��ʵ�鲽�����£�
a.����Ӧ��ȡ�������ƹ��������Ϊ________g��
b.�����������ƹ��塣
c.���ձ��е���Һע������ƿ��������������ˮϴ���ձ��ڱ�2~3�Σ�ϴ��ҺҲע����
��ƿ��
d.������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e.�Ǻ�ƿ�����������µߵ���ҡ�ȡ�
f.����������ƿ�м�����ˮ���̶�����1~2cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����
�̶������С�
�����������ȷ����˳��Ϊ_______________________������ű�ʾ����
�ܸ��ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ
mL�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��á�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com