
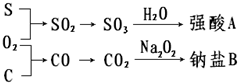 �������ʼ�����ͼ��ʾת����ϵ���ش��������⣺
�������ʼ�����ͼ��ʾת����ϵ���ش��������⣺

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
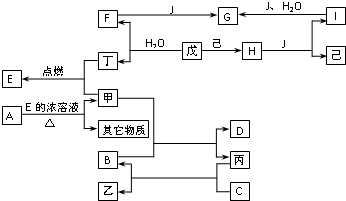 ��֪A��B��C��D��E��F��G��H��I��J�ǻ�����ס��ѡ����������졢���ǵ��ʣ����У�F��H��G��I��ɫ��Ӧ����ʻ�ɫ��B��C��D��ɫ��Ӧ���ܲ����۲�������ɫ��H�ǵ���ɫ���壮�����ʼ�����ͼ��ʾ��ת����ϵ��
��֪A��B��C��D��E��F��G��H��I��J�ǻ�����ס��ѡ����������졢���ǵ��ʣ����У�F��H��G��I��ɫ��Ӧ����ʻ�ɫ��B��C��D��ɫ��Ӧ���ܲ����۲�������ɫ��H�ǵ���ɫ���壮�����ʼ�����ͼ��ʾ��ת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004ȫ����ʡ�и߿�ģ�������ࡤ��ѧ ���ͣ�043
����Aֻ����X��Y���ֶ�����Ԫ�أ�X��ԭ����������Y��ԭ��������B��D��E����ѧ��ѧ�г������壬�����ʼ�����ͼ��ʾ��ת����ϵ��
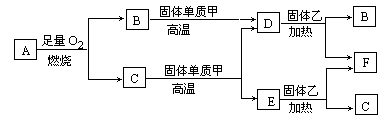
�Իش��������⣺
(1)������A�У�X��Y��Ԫ�ص������ȵ���3��1����д���������ʵĻ�ѧʽ��
A��________��B��________��D��________��
F��________���ң�________��
(2)��X��Y��Ԫ�ص�����������ֵ�ϵ���X�����ԭ��������������A�ķ���Ϊֱ���ͽṹ����A�ĵ���ʽΪ________��
(3)C�������ڸ���ʱ������Ӧ�Ļ�ѧ����ʽΪ��
________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ��ƶ���

 B(s)+D(g) ��H=akJ/mol(a>0)�����¶���ƽ�ⳣ��K=0.263��������1 mol B�������յ�����____��ѡ����ڡ��������ڡ���С�ڡ���a kJ�������������A����C��ת����__ ��ѡ����ߡ��������䡱���͡�������������ѹǿ������ʱ��仯����÷�Ӧ__ ��ѡ��ﵽ������δ�ﵽ����һ���ﵽ������ѧƽ��״̬���÷�Ӧ�ﵽ��ѧƽ��״̬ʱ����c( C) =0. 100 mol/L����c(D)=___mol/L��
B(s)+D(g) ��H=akJ/mol(a>0)�����¶���ƽ�ⳣ��K=0.263��������1 mol B�������յ�����____��ѡ����ڡ��������ڡ���С�ڡ���a kJ�������������A����C��ת����__ ��ѡ����ߡ��������䡱���͡�������������ѹǿ������ʱ��仯����÷�Ӧ__ ��ѡ��ﵽ������δ�ﵽ����һ���ﵽ������ѧƽ��״̬���÷�Ӧ�ﵽ��ѧƽ��״̬ʱ����c( C) =0. 100 mol/L����c(D)=___mol/L�� �鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com