
(10��) ��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�
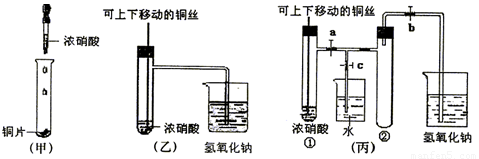
��1�� д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ���
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ��,��ʵ��ʱ�ȹرյ��ɼ�
���ٴ��ɼ�
������ʹNO2����������Թܡ�
��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�
�� ��
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
�������������״�����㣩��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��) ��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�
��1�� д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ���
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ��,��ʵ��ʱ�ȹرյ��ɼ�
���ٴ��ɼ� ������ʹNO2����������Թܡ�
��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�
�� ��
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
�������������״�����㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09��10��ɶ�ʯ����ѧ��һ������ĩ���Ի�ѧ�� ���ͣ�ʵ����
(10��)��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�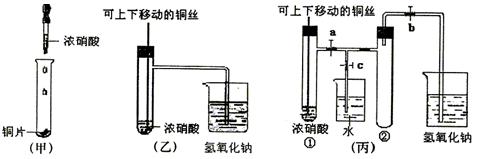
��1�� д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ���
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ��,��ʵ��ʱ�ȹرյ��ɼ�
���ٴ��ɼ� ������ʹNO2����������Թܡ�


��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�
�� ��
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
�������������״�����㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡͭ��һ�и߶�6���¿���ѧ�Ծ����������� ���ͣ������
(10��)��ͼ�Ǻϳ�һ�ָ߷��ӵIJ�������,��Ӧ����һ�������½���,ijЩ��N�ӻ��Ļ��������������ڱ���������գ�
��1�����뱽�ӵĹ�ϵ�ǣ� ��ѡ����ĸ��ţ���
A����Ϊͬϵ�� B����Ϊͬ���칹�� C��ͬ�������廯���� D������������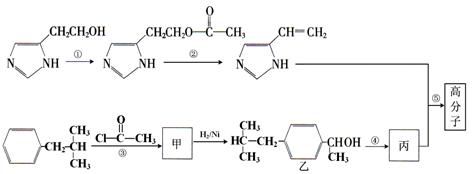
��2��д����Ӧ���͢� ��
��3��д����ȥ��Ӧ�ڵĻ�ѧ����ʽ������ע����Ӧ������:
��
��4��д���Ӿ۷�Ӧ�ݵĻ�ѧ����ʽ������Ӧ������ʵ���Ϊ1��1������ע����Ӧ������
��
��5������˵����ȷ���� ��ѡ����ĸ��ţ���
A�������������������
B�������ʼ���ʹ���Ը��������Һ��ɫ������ʹ���CCl4��Һ��ɫ��
C���ҷ��ӵ�1H-NMR���˴Ź������ף������ַ�������Ϊ6:3:2:2:2:1:1:1
D����������������Ʒ�Ӧ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08�����а�У����)
����(4��) �����йػ�ѧʵ���˵����ȷ���ǣ�����ţ� ��
A���ζ��õ���ƿ�͵ζ��ܶ�Ҫ����ʢ��Һ��ϴ
B���Ʊ�(�ռ�)�κ����忪ʼʵ��ǰ����Ҫ���ȼ��װ�õ�������
C������ѧ������ͭ������ᾧˮ�����ⶨ����ʵ���У���������������Ҫ�Ĵ�
D���ⶨ��Һ��pHʱ��Ӧ�Ƚ�pH��ֽ��ʪ
E������������Һʱ����������Ͳ�ڼ���һ�������ˮ�����ڽ�������������Ũ����
F��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ������ʪ������
����11�֣���ͼ���Ǿɽ̲�����֤ͭ��Ũ���ᷴӦ��װ�ã������½̲Ķ���ʾʵ��Ľ����װ�ã�����ijУʦ����������һ���Ľ���������������������ʽ���̽����װ�á�
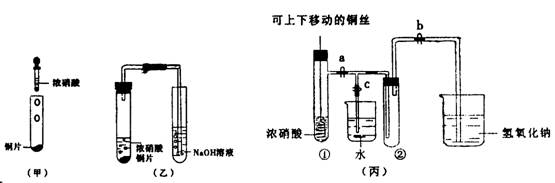
��1�����װ����ȣ���װ�õ��ŵ��� �� ��װ�ó�����װ���ŵ�����е��ŵ��� ����д��һ�㼴�ɣ�
��2����ʹNO2��������Թܢڣ�Ӧ�ȹرյ��ɼ� ���ٴ��ɼ� ������������ں�ͭ˿��������Һ���룬Ȼ���a��b��c���رգ������Թܢ����ڷ�ˮ�У��������������� ��
A����ɫ����B��ƽ��Ħ������ ��C��ѹǿ�� D���ܶ�
��3��Ϊ����֤NO2��ˮ�ķ�Ӧ����ʹ�ձ��е�ˮ�����ԹܢڵIJ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com