
(12��)��1���õ���ʽ��ʾH2O��MgBr2���γɹ���
H2O MgBr2
��2��д��CO2 ��Na2O2��H2O2�ĵ���ʽ��
CO2 Na2O2 H2O2
��3�� H2O�� ����ϣ�MgBr2�� ����ϡ�NaOH�� ����ϣ�Na2O2�� ����ϣ���Լ����Ǽ��Լ������Ӽ���
��4��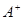 ��
��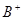 ��
��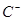 ��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����
��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����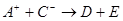 ����
����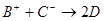 ��
��
�ݴˣ��ش��������⣺
��д���ٷ�Ӧ�����ӷ���ʽ ��
��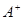 ��C-�ĵ���ʽ
��C-�ĵ���ʽ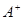 ____________��C-____________��
____________��C-____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)��1���õ���ʽ��ʾH2O��MgBr2���γɹ���
H2O MgBr2
��2��д��CO2 ��Na2O2��H2O2�ĵ���ʽ��
CO2 Na2O2 H2O2
��3�� H2O�� ����ϣ�MgBr2�� ����ϡ�NaOH�� ����ϣ�Na2O2�� ����ϣ���Լ����Ǽ��Լ������Ӽ���
��4��![]() ��
��![]() ��
��![]() ��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����
��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����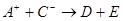 ����
����![]() ��
��
�ݴˣ��ش��������⣺
��д���ٷ�Ӧ�����ӷ���ʽ ��
��![]() ��C-�ĵ���ʽ
��C-�ĵ���ʽ![]() ____________��C-____________��
____________��C-____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ������ʡ������·ʵ����ѧ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ������
(12��)��1���õ���ʽ��ʾH2O��MgBr2���γɹ���
H2O MgBr2
��2��д��CO2��Na2O2��H2O2�ĵ���ʽ��
CO2 Na2O2 H2O2
��3�� H2O�� ����ϣ�MgBr2�� ����ϡ�NaOH�� ����ϣ�Na2O2�� ����ϣ���Լ����Ǽ��Լ������Ӽ���
��4��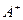 ��
��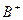 ��
��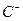 ��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����
��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����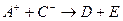 ����
���� ��
�� �ݴˣ��ش��������⣺
�ݴˣ��ش��������⣺
��д���ٷ�Ӧ�����ӷ���ʽ ��
�� ��C-�ĵ���ʽ
��C-�ĵ���ʽ ____________��C-____________��
____________��C-____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��9�֣�A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��Dͬ���壻C��Eͬ���壻B��Cͬ���ڣ�Bԭ���������������������������2��A�����ڱ��а뾶��С��Ԫ�أ�C���������������ڲ���ӵ�3����FԪ����ͬ����Ԫ����ԭ�Ӱ뾶��С������Ԫ�ء�A��B��C��D��E�γɵĻ�����ס��ҡ���������������±���ʾ��
|
������ |
�� |
�� |
�� |
�� |
|
��ѧʽ |
A2C |
A2C2 |
D2C2 |
D2E |
�ش��������⣺
��1���õ���ʽ��ʾ�γɻ����ﶡ�Ĺ��� ��
��2��д�������Ӧ�Ļ�ѧ����ʽ��
(3)����������ȼ�ϵ�����Թ�������ﯡ�������Ϊ����ʣ����ֹ��������ڸ���������O2-�����ͨ�����õ�صĹ���ԭ������ͼ��ʾ�����ж��Pt�缫a��b�ֱ�������C2��A2�����塣��O2-���� �������������������
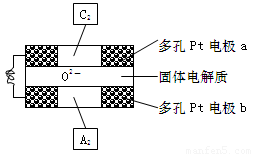
�ڸõ�ص�������ӦΪ ��������ӦΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com