
��Ҫ����ˮ���Ȼ�þ(![]() )Ϊԭ���Ʊ���ˮ�Ȼ�þ����֪��
)Ϊԭ���Ʊ���ˮ�Ȼ�þ����֪��![]() �����ڿ����м���ʱ�����ͷų����ֽᾧˮ��ͬʱ����
�����ڿ����м���ʱ�����ͷų����ֽᾧˮ��ͬʱ����
Mg(OH)Cl��MgO���ڸ����HCl�����м����Ƶ���ˮ![]() ����ѡ�õ�ҩƷΪ��
����ѡ�õ�ҩƷΪ��![]() ���壬NaCl(��)��
���壬NaCl(��)��![]() (��)��
(��)��![]() ��Ũ
��Ũ![]() ��Ũ���ᣬNaOH��Һ������װ�ü�ͼ��
��Ũ���ᣬNaOH��Һ������װ�ü�ͼ��
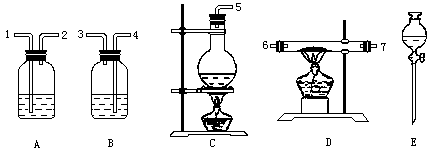
(1)��װHCl���巢����ʱ��Ӧѡ�õ�װ����(��д���)________����Ӧ����ʽ��________��
(2)�����������Ǵ������ң������������ӿڵ�����˳��(��д����)��________��
(3)��װ����Ӧ�ŵ�ҩƷ�ǣ�A________B________C________D________E________��
(4)Bװ�õ�������________��
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com