
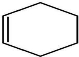
 ��C��ͬ���칹���в�������a��ѡ����ţ���
��C��ͬ���칹���в�������a��ѡ����ţ������� ��1������̼̼˫�����������4��C��̼̼˫������2��3��C�м䣬2��3��C����2������
��2��BΪ��CH3��2CBrCBr��CH3��2���������NaOH���Ҵ���Һ���ȿ����ɶ�ϩ����Ϊ��ȥ��Ӧ������CΪCH2�TC��CH3��-C��CH3���TCH2��
��3��C��һ����Br2��Ӧ��������D��E��G����D��HBr�ļӳɲ���ֻ��F��˵��D�ṹ�Գƣ�C����1��4�ӳɣ���ȷ��F�Ľṹ��ʽ��
��4��C��һ��ͬ���칹��H������KMnO4��Һ���ɼ����ᣬ��HΪ����ϩ��
��� �⣺��1������̼̼˫�����������4��C��̼̼˫������2��3��C�м䣬2��3��C����2������A��ϵͳ������Ϊ2��3-����-2-��ϩ��������ΪҺ�壬
�ʴ�Ϊ��2��3-����-2-��ϩ��Һ��
��2��BΪ��CH3��2CBrCBr��CH3��2���������NaOH���Ҵ���Һ���ȿ����ɶ�ϩ����Ϊ��ȥ��Ӧ������CΪCH2�TC��CH3��-C��CH3���TCH2��
�ʴ�Ϊ��CH2�TC��CH3��-C��CH3���TCH2����ȥ��Ӧ��
��3��C��һ����Br2��Ӧ��������D��E��G����D��HBr�ļӳɲ���ֻ��F��˵��D�ṹ�Գƣ�C����1��4�ӳɣ���FΪCH2BrCBr��CH3��-CH��CH3��CH2Br��
�ʴ�Ϊ��CH2BrCBr��CH3��-CH��CH3��CH2Br��
��4��C��һ��ͬ���칹��H������KMnO4��Һ���ɼ����ᣬ��HΪ����ϩ���� ����Ӧ��ͬ���칹�����Ϊȷ������ϩ���Լ���ϩ����������Ϊ���ȣ�
����Ӧ��ͬ���칹�����Ϊȷ������ϩ���Լ���ϩ����������Ϊ���ȣ�
�ʴ�Ϊ�� ��a��
��a��
���� ���⿼�����л�����ƶϣ�Ϊ��Ƶ���㣬�������ȴ���ȡ����Ӧ����ȥ��Ӧ�Ŀ��飬ע������л���Ľṹ�����ŵ����ʣ��Ѷ��еȣ�ע�ⲻͬ�����л����ͬ���칹����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Һ[Ba��OH��2��Һ] | B�� | FeSO4��Һ��KSCN��Һ�� | ||
| C�� | KI��������Һ�� | D�� | Na2SO3��Һ��BaCl2��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C | B�� | Fe | C�� | Cu | D�� | Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3.20g | B�� | 10.22g | C�� | 12.62g | D�� | 13.70g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ̿�������� | ||
| C�� | ������ӦʽΪFe3++e-��Fe2+ | D�� | ������ӦʽΪFe2+-e-��Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10B��10B20��Ϊͬ�������� | B�� | BԪ�ص���������10 | ||
| C�� | 10B���������ͺ����������� | D�� | 10B20�����۵�ߡ�Ӳ�ȴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Cl2ͨ��ˮ���γɵ���Һ������ Cl2+H2O�T2 H++Cl-+ClO- | |
| B�� | ���۵⻯����Һ�ڿ����б��� 4 I-+O2+2 H2O�T2 I2+4 OH- | |
| C�� | ���������ˮĤ���Խ�ǿʱ��ˮĤ�е�H+�ᱻ��ԭ O2+4 H++4 e-�T2 H2O | |
| D�� | ��Na2SiO3��Һ��ͨ�����SO2���н�״��������SiO32-+SO2+H2O�TH2SiO3��+SO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ�Ũ��֮��c��A����c��B����c��C��=2��3��4 | |
| B�� | ��������и����ʵ�Ũ����� | |
| C�� | ��λʱ���ڣ���������a molA���ʣ�ͬʱҲ������2a mol C���� | |
| D�� | ������������Ƿ�Ӧ��ʼǰ��$\frac{4}{5}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ũ���ᡢŨ�����Ϲ�����ȡ�������Ƿ���ȡ����Ӧ | |
| B�� | C5H10������������C5H12����������������ͬ���칹�� | |
| C�� | ���ۺ���ά�ؾ����ã�C6H10O5��n��ʾ��������ǻ�Ϊͬ���칹�� | |
| D�� | ���ᡢ��������һ�������¾�����Cu��OH��2��Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com