
| һ������ױ� | 234�� | 206�� | 213.8�� | 204�� | 214.5�� | 205 |
| ��Ӧ�Ķ��ױ� | -13�� | -54�� | -27�� | -54�� | -27�� | -54�� |
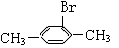 ��2���� ��
��2���� �� �� 3��3-����-1-��ϩ
�� 3��3-����-1-��ϩ
 ��
�� XCO2+Y/2H2O����̬����(X+Y/4)ԽС��������ԽС���������ʵ�������������������������С��Ϊ�ۣ�����1molO2ʱ����Ҫ12gC��4gH��������ͬ������������ȫȼ�գ����к�C%Խ�ߣ�����O2Խ�٣���������ʱ������������Ϊ�١�
XCO2+Y/2H2O����̬����(X+Y/4)ԽС��������ԽС���������ʵ�������������������������С��Ϊ�ۣ�����1molO2ʱ����Ҫ12gC��4gH��������ͬ������������ȫȼ�գ����к�C%Խ�ߣ�����O2Խ�٣���������ʱ������������Ϊ�١� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��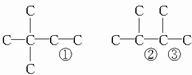 ����A�����еĽṹ��ʽΪ��CH3��3C-CH=CCH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2�ȣ�
����A�����еĽṹ��ʽΪ��CH3��3C-CH=CCH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2�ȣ� nCO2+(n-1)H2O����̬����������O2�������ͬ״���¸������������8.5������(3n-1)/2=8.5�����n=6����A��C6H10��
nCO2+(n-1)H2O����̬����������O2�������ͬ״���¸������������8.5������(3n-1)/2=8.5�����n=6����A��C6H10�� �ȡ�
�ȡ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HF��HI���ȶ�����Ҫԭ����HF����֮�����γɽ�ǿ����� |
| B�����쵰�ס���˿����������ø����֬���ˮ�����ɵõ������� |
| C����������Ӽ������������Ӽ����ۼ���Ϊ������������ |
| D�����ϣȼ�շ������۷���ͭ˿ȼ�շ���ֽ����������Ԫ�ض��Է����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ҵ� | B���Ҷ��� | C����ϩ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��(3b��0.5a) mol | B��(4b��0.5a) mol |
| C��(3b��1.5a) mol | D�����ж� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com