
(1)�����л������ͨʽ�����и�ʽ�е�__________(����ĸ����)��
A.Cn(H2O)m B.(C2H3)n(H2O)m
C.(C2H)n(H2O)m D.(CH)n(H2O)m
E.(C2O)n(H2O)m F.(CO)n(H2O)m
(2)�����ϸ�ͨʽ��ij�л������Է�������Ϊ136�����仯ѧʽΪ__________�������л������������ҷ����к��б�����������ܵ�ͬ���칹����__________�֣�д�������������ֵĽṹ��ʽ��____________________��____________________��
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��1�������л�������ͨʽ���������е���ʽ����A��B��C��գ���
A��(Cx)n(H2O)m
B��(CxHy)n(H2O)m
C��(CxOy)n(H2O)m
�����ͨʽΪ________���������е�ԭ�Ӹ������廯����x=1��2��3��������
��2�������л����У�����Է�������Ϊ136�������ʽΪ________��
��3���������л����к��б�������д���������������ͬ���칹��Ľṹ��ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
���ȼ��ֻ��̼���⡢����һ���л��������������������ɶ�����̼�����֮��Ϊ9�U8����ͬ�����£�
��1�������л�������ͨʽ���������е���ʽ����A��B��C��գ���
A��(Cx)n(H2O)m
B��(CxHy)n(H2O)m
C��(CxOy)n(H2O)m
�����ͨʽΪ________���������е�ԭ�Ӹ������廯����x=1��2��3��������
��2�������л����У�����Է�������Ϊ136�������ʽΪ________��
��3���������л����к��б�������д���������������ͬ���칹��Ľṹ��ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ƺ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��֪ij��̬��ʯȼ��X��ֻ����̼��������Ԫ�أ�Ϊ̽����������̼��������Ԫ�ص������ȣ�ijͬѧ�����ȼ�շ�������ʵ�鷽����ͨ������װ��C��D�����ؼ������̼��������Ԫ�ص������ȡ�ʵ��װ����ͼ��ʾ(��֪CuO������Ϊ̼�⻯����ȼ�յĴ���)��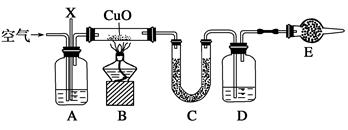
(1)��Aװ������ʢ�ŵ�ҩƷ��ŨNaOH ��Һ��д��װ��A��һ�����ã�_
(2)Cװ������ʢ�ŵ�ҩƷ�ǣ�
(3)Dװ������ʢ�ŵ�ҩƷ�ǣ�
(4)Eװ������ʢ�ŵ�ҩƷ�ǣ�
(5)����װ������һ������ (������
�ƾ��ƺͼ��ȷ������ܴ��ڵĴ���
������������ҩƷ����ָ��������ҩ
Ʒ���ƺ�λ��)�������������
(6) ��ʵ��װ�þ��������������¶���ʵ�飺ȷ��ȡ7.2 g��Ʒ��ֻ��C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�U�ι�C����������10.8 g�����ƿD��������22 g������л�������ʽΪ
��7������ȷ�������ʽ������ͬ���칹���зе�������ʵ�����__ �� (ϰ��������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��5 4.2������ϰ���������棩 ���ͣ������
A��B��C��D����ֻ����̼���⡢������Ԫ�ص��л���ڳ�����AΪ��̬��B��CΪҺ̬��D�ǰ�ɫ���壮A��B����ǿ�ҵĴ̼�����ζ��C�й���ζ��D����ζ�����Ǿ�����ͬ��ʵ��ʽ���ֱ������������г��ȼ�պָ��������£���ȼ������O2�����ʵ�����ȼ�ղ�������������ʵ�����ȣ�����������⣺
(1)�����л����ʵ��ʽ��________��
(2)д���������ʵĽṹ��ʽ��A______________��B______________��C____________��D____________.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com