
| m |
| M |
| 1g |
| 34g/mol |
| 1 |
| 34 |
| 256.652KJ |
| 0.4 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ݶԽ��߹�������������ʾ��������� |
| B��[Cu��H2O��4]2+��Cu�ṩ�չ����H2O��O�ṩ�¶Ե����γ���λ�� |
| C��Ԫ�ص縺��Խ���ԭ�ӣ��������ӵ�����Խǿ |
| D�����Է��ӻ�Ϊ�������ǵ�����û������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
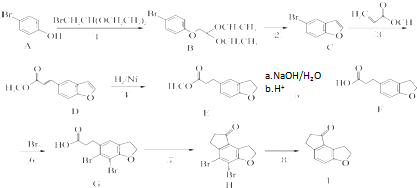
| PDC/DMF |
 Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ�����Լ���ѡ�������ϳ�·�߳��õı�ʾ����Ϊ��A
�ĺϳ�·������ͼ�����Լ���ѡ�������ϳ�·�߳��õı�ʾ����Ϊ��A| ��Ӧ�Լ� |
| ��Ӧ���� |
| ��Ӧ�Լ� |
| ��Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��ԭ�Ӱ뾶Z��M |
| B��Zλ��Ԫ�����ڱ��е�2���ڡ��ڢ�A�� |
| C��X�������̬�⻯������ȶ��Ա�Z��ǿ |
| D��Y������������Ӧˮ��������Ա�X��ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� | X | Y | Z |
| ����Ԫ������ | 1 | 2 | 3 |
| �����еĵ������� | ����10 | 18 | |
| ȼ���ȣ�kJ/mol�� | -285.8 | -283 | -726.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
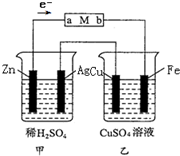 ����װ����ij�С�����ʵ���װ��ͼ���ش��������⣮
����װ����ij�С�����ʵ���װ��ͼ���ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������������ᷴӦ������Ӧ |
| B������Na2SO4��Һ����ʹ�����ʱ��� |
| C����֬�����������������ijЩά���ص����� |
| D�����ǡ���֬����������һ�������¾�����ˮ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com