
 ����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У�
����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У�| 52 |
| 52+16��3 |
| 52 |
| 52 |
| 42.67 |
| 16 |
| 52 |
| 52 |
| 24 |
| 16 |
| ||
| ||
| 3 |
| ||
| 3 |
| ||
| 3 | 2.0��10-25 |
| KspCr(OH)3 |
| C3(OH-) |
| 6.0��10-31 |
| 2.0��10 -25 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
�����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�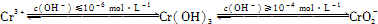
2- 7 |
2- 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ��������⣨������ѧ�Ծ��������棩 ���ͣ������
��11�֣�����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�
��1��CrO3����ǿ�����ԣ������л����ƾ���ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ��
____________ ��
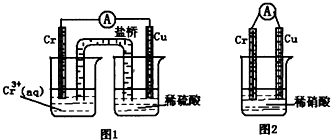
��2��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ��
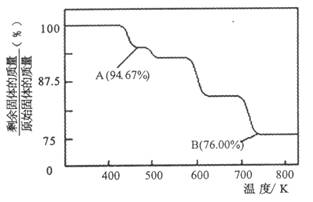
��A ��ʱʣ�����ijɷ���_________ ���ѧʽ����
�ڴӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ______________________ _��
��3��CrO3�� K2Cr2O7��������ˮ�����ǹ�ҵ����ɸ���Ⱦ����Ҫԭ������������֮һ�ǽ�����6�� Cr �ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2+��Cr2O72��������Ӧ�����ɵ�Fe3+��Cr3+����������OH��������� Fe(OH)3 ��Cr(OH)3������ȥ[��֪ KspFe(OH)3��4.0��10��38��KspCr(OH)3��6.0��10��31]��
�ٵ������� NaCl ��������__________________________��
����֪�������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵ�һ��ͳ����ѧ�Ծ� ���ͣ������
(16��)������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�
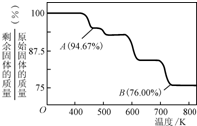
��1������ͼװ���У��۲쵽ͼ1װ��ͭ�缫�ϲ�����������ɫ���ݣ���ͼ 2װ����ͭ�缫����������������缫�ϲ���������ɫ���塣��ͼ 1 ֪�������Ļ�Ա�ͭ_____(��ǿ����)��ͼ 2װ���и��缫�ĵ缫��Ӧʽ

��2��CrO3����ǿ�����ԣ������л����ƾ���ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬ CrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________________��
(3)����ƽ�⣺2CrO42������ɫ��+2H+ Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O
����ƽ����ϵ��pH=2������Һ�� ɫ.
����˵���ڢٲ���Ӧ��ƽ��״̬���� ��
a��Cr2O72����CrO42����Ũ����ͬ b��2v (Cr2O72��) =v (CrO42��) c����Һ����ɫ����
��4��CrO3�� K2Cr2O7��������ˮ�����ǹ�ҵ����ɸ���Ⱦ����Ҫԭ������������֮һ�ǽ�����6�� Cr �ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2+��Cr2O72��������Ӧ�����ɵ�Fe3+��Cr3+����������OH��������� Fe(OH)3 ��Cr(OH)3������ȥ[��֪ KspFe(OH)3��4.0��10-38��KspCr(OH)3��6.0��10-31]��
�ٵ������� NaCl ��������__________________________��
����֪�������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L1������Һ��c(Cr3+)Ϊ____ mol��L-1��
��5��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ��

�ӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com