
ijУ��һ��ѧ�о���ѧϰС����ճ������е�������;�����˵��飬�˽�����ɹ㷺��Ӧ���������Ư�ס�ˮ���ɱ���������ȡ�
��1��������������Ư�ס�������ԭ������Ϊ����ˮ�������ɾ���Ư�ס��������õ�_________����ط�Ӧ�����ӷ���ʽΪ__________________��
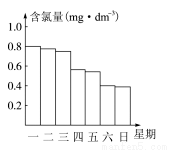
��2���ڵ���ij������Ӿ���ļ���ˮ�������ʱ��С���Ա�˽������Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ������������ͨ����Ӿ����ˮ�ĺ���������Ч�ȣ�������0.5 mg��dm��1��1.0 mg��dm��1֮��ʱ��Ч����á���ͼ�Ǹ�С��ⶨ��ÿ��19��00ʱ��Ӿ����ˮ�ĺ��������ļ���ʹ����Ӿ�ز���ȫ��________________��
��3������Ϊ�ļ�����������ȡ�����ǿ��____________��˵��һ������__________����Ҫ�ķ���ʽ�����֣���
��4��С����Ӿ��ͨ��ʹ��Ư��Һ��NaClO��Һ������������������ˮ���Ծٳ�ʹ��Ư��Һ��������������һ������_________________���û�ѧ����ʽ˵����ҵ���������Ư��Һ��_________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ��һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Ϊ�˳�ȥ�����е� Ca2+��Mg2+��SO42-����ɳ���ɽ���������ˮ��Ȼ������������������������ȷ�IJ���˳����
�ٹ���
�ڼӹ���NaOH��Һ
�ۼ���������
�ܼӹ��� Na2CO3��Һ
�ݼӹ��� BaCl2��Һ
A���٢ܢڢݢ� B���ܢ٢ڢݢ� C���ڢܢݢ٢� D���ݢڢܢ٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ��ʡ��һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�������
ʵ���ҳ����������������Ϊ36.5%���ܶ�Ϊ1.20g/�M3��
��1����Ũ��������ʵ���Ũ���Ƕ��٣�(��ʽ����)
��2������100mL3.00mol/L�����ᣬ������Ũ�������mL ��(��ʽ����)
��3����Ũ�������Ƹ�ϡ������Ҫ������Щ���裨������˳����д��ţ��� ��
�ټ��� ��װƿ����50 mL��Ͳ��ȡһ�������Ũ�����ϴ�Ӣ���Һ��ϡ�͢߶��ݢ�ҡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�츣��ʡ������ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʼ�ת������ͨ��һ��ʵ�ֵ���
A��Si��SiO2��H2SiO3��Na2SiO3
B��Al��Al2O3��NaAlO2��Al(OH)3
C��S��SO3��H2SO4��SO2
D��N2��NO2��HNO3��NO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ�����и�����ѧ�����м�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
25��ʱ�������й���Һ���������ʵ���Ũ�ȹ�ϵ��ȷ����
A��pH=2��CH3COOH��Һ��pH=12��NaOH��Һ�������ϣ�
c(Na��)��c(CH3COO��)��c(OH��)��c(H��)
B��0.1 mol��L-1CH3COONa��Һ��0.1 mol��L-1CH3COOH��Һ�������ϣ�pH=4.75��:
c (CH3COO��) + c (CH3COOH) = 2 c(Na��)
C��0.1 mol��L-1CH3COONa��Һ��0.1 mol��L-1 HCl��Һ�����pH=7��
c(Na��)��c(Cl��) = c(CH3COOH)��c(CH3COO��)
D��0.1 mol��L-1 Na2CO3��Һ��0.1 mol��L-1 NaHCO3��Һ��������:
c (HCO3-) + 2c (H��) + 3c (H2CO3) = c(CO32-) + 2c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�긣��ʡ�����и�һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵı��淽������ȷ����
A����ˮ��������ɫ�Լ�ƿ�� B��Ư��¶���ڿ����д��
C����������Ӧ�ܷⱣ�� D�������Ʊ�����ú����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꼪��ʡ�»��и�һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͬ��ͬѹ�£�������������������ȷ����
A��8gO2��1gH2���Ϊ1:2
B��NH3��H2S�ܶȱ�Ϊ1:2
C��5L N2��4L CH4ԭ������Ϊ1:2
D��2molCO2��1molCO��������Ϊ1:2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꼪��ʵ����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��15g���ֽ����Ļ����Ͷ�������������У���Ӧ��ȫ��õ�11.2L H2 (��״��)���û�������ɿ����ǣ� ��
A���ƺ��� B��þ��ͭ C������þ D��п����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�갲��ʡ��һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��������Ħ��������2g B��Ħ�������ʵ�������λ
C��1molOH����������17g D��1mol������ռ�����ԼΪ22.4L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com