



 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
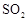 ת��Ϊ
ת��Ϊ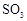 ��ת���ʲⶨʵ�飺
��ת���ʲⶨʵ�飺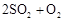
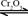
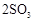 ��
��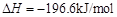 ����֪��
����֪��| | �۵㣨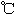 �� �� | �е㣨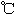 �� �� |
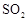 | -72.4 | -10 |
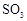 | 16.8 | 44.3 |
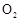 ��Ϊʹ
��Ϊʹ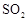 �нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��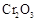 �������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������
�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������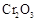 ���ķ�Ӧ��ʱ��
���ķ�Ӧ��ʱ��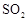 ��ת���ʻ� ������ߡ��������͡����䡱����
��ת���ʻ� ������ߡ��������͡����䡱����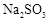 �������Ũ�����������ģ���Ӧ����ʱ����ͨ��
�������Ũ�����������ģ���Ӧ����ʱ����ͨ��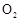 һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ����
һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ����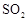 ��ת����Ϊ %������С�����һλ����
��ת����Ϊ %������С�����һλ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ũ�����Ũ���ᶼ������������������ʢװ |
| B��Ũ�����Ũ���ᶼ���к�ǿ�ĸ�ʴ�ԡ���ˮ�� |
| C��ϡ�����ϡ���ᶼ���������� |
| D����������ᶼ����Ҫ�Ļ���ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ó���ʯ��ˮ����SO2��CO2 |
| B������ڹ����Ĵ�����ȼ�տ�������SO3 |
| C��SO2��ʹ��ˮ������KMnO4��Һ��ɫ |
| D������SO2ͨ��Ũ��CaCl2��Һ�����ɰ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ɫ | B������ɫ | C������ɫ | D������ɫ���ָ�ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʹƷ����ɫ�� |
| B����ʹʪ�����ɫʯ����ֽ��졣 |
| C��ͨ��������NaOH��Һ�У��ٵ���BaCl2��Һ�а�ɫ�������ɣ��ó�������ϡ���ᡣ |
| D��ͨ��ϡ��ˮ����ʹ��ˮ��ɫ���ó�����Һ���ٵμ�Ba(NO3)2��Һ�а�ɫ�������ó������������ᡣ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ȼ��� | B�������� | C�������� | D��̼���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com