
����Ŀ��ij���dz��Ը���Ϊԭ�����ǣ�ͬʱ�õ������ĸ��������Ը�������ۺ����ã�����������߾���Ч�棬���һ��ܷ�ֹ������Ⱦ���ְ����·�ʽ���У�

��֪F��H���Ǿ�����ζ��Һ�壬FΪE�����ۺ�������������Ԫ��״�Գƽṹ������գ�
��1��A������________��D��ͬ���칹��Ľṹ��ʽΪ________________��
��2��E��G�Ļ�ѧ����ʽ_________________________��
��3��G��H�Ļ�ѧ����ʽ_________________________��
��4��F�Ľṹ��ʽ��_____________________��
���𰸡� ��ά�� CH3��O��CH3  CH3CH2OH+CH3COOH
CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O 
��������������A�ķ���ʽ��C6H10O5��n��֪A���ǵ��۾�����ά�أ���ϵ����ʵ��ȷ��AΪ��ά�أ�BΪ�����ǣ�DΪ�ƾ���EΪ��ȩ��FΪE��������Ҿ����������Ԫ��״�Գƽṹ���������ݳɼ�ԭ��ֻ����C=O��ʽ�ӳ����ɡ���ͼ��ʾ�� ����1��A��������ά�أ�DΪ�ƾ�����ͬ���칹��Ľṹ��ʽΪCH3��O��CH3����2��E��G����ȩ�����������ᣬ��Ӧ�Ļ�ѧ����ʽΪ��
����1��A��������ά�أ�DΪ�ƾ�����ͬ���칹��Ľṹ��ʽΪCH3��O��CH3����2��E��G����ȩ�����������ᣬ��Ӧ�Ļ�ѧ����ʽΪ��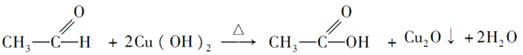 ��
��
��3��G��H������Ҵ���Ũ����Ĵ��·�Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O����4��F�Ľṹ��ʽΪ��
CH3COOCH2CH3+H2O����4��F�Ľṹ��ʽΪ�� ��
��


 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�������ķ���ʽΪC18H26O5��1mol������ȫˮ��ɵõ�1mol�����2mol�Ҵ���������ķ���ʽΪ
A.C14H18O5B.C14H16O4C.C14H22O5D.C14H10O5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ҫ�Ŀ�����Ⱦ����Ŀǰ��������������Ⱦ�ķ����ж��֡�
(1)��CH4����ԭ��������������������������Ⱦ��
CH4(g)��4NO(g)��2N2(g)��CO2(g)��2H2O(g) ��H����1160 kJ��mol��1������
CH4(g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H����574 kJ��mol��1������
H2O(g)��H2O(l) ��H����44��0 kJ��mol��1������
��д��CH4 (g)��NO2 (g)��Ӧ����N2 (g) ,CO2(g)��H2O(l)���Ȼ�ѧ����ʽ��________
��Ϊ�о���ͬ�����¶�������Ӧ( �� )��Ӱ�죬�ں���������,��2 L �ĺ����ܱ������м���0.2mol CH4��0.4mol NO2,10min��Ӧ�������ﵽƽ�⣬���l0min��v(NO)=5��10-3mol/(L��min),��ƽ���n(CH4)=___mol,NO2��ת����a1=_________.�����������䣬��Ӧ�ں�ѹ�����½��У�ƽ��ʱNO2��ת����a2____a1(����ڡ� С�ڡ��� ���ڡ� )��
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)��2NO(g)![]() N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
ʱ�� Ũ��(mol/L) ���� | NO | N2 | CO2 |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
40 | 0.032 | 0.034 | 0.017 |
50 | 0.032 | 0.034 | 0.017 |
�ٲ�����Ϊ�жϷ�Ӧ�ﵽ��ѧƽ��״̬������ ��_________
A��������CO2��Ũ�ȱ��ֲ���
B��v����N2��= v����NO��
C����������ƽ����Է����������ֲ���
D�����������ܶȱ��ֲ���
E��������ѹǿ���ֲ���
����T��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ_______ (������λС��)��
����30 min,�ı�ijһ����,��Ӧ���´ﵽƽ��,��ı��������________��
��3����ѧ�������о����ô������������ٷɻ�β���е�NO��COת���CO2��N2, �о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ���ͼ��ʾ��������������ʱ����Ӧ��2CO(g)��2NO(g)![]() N2(g)��2CO2(g) ��NO��Ũ��c(NO)���¶�(T)�����������(S)��ʱ��(t)�ı仯���ߡ�
N2(g)��2CO2(g) ��NO��Ũ��c(NO)���¶�(T)�����������(S)��ʱ��(t)�ı仯���ߡ�

�ٸ÷�Ӧ�Ħ�H____0���>����<������
���������ı����S1 >S2����ͼ�л���c(NO)��T1��S2�����´ﵽƽ������еı仯���ߡ�____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڼ�����õ�FeSO4��Һ�Ƿ���ʵ��Լ�
A.��������B.����C.����D.KSCN��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���������а��̳��ֵ��ǣ� ��
A.ͭ˿��Cl2��ȼ��
B.��˿��������ȼ��
C.������������ȼ��
D.Na��Cl2��ȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���輰�仯�����Ӧ�÷�Χ�ܹ㡣����˵����ȷ����
A. �������ཫ̫����ת��Ϊ���ܵij��ò���
B. �ֹ��Ʊ������費�漰������ԭ��Ӧ
C. ʯӢ�����Ͳ����ϵĴ��̶��ǹ�������Ʒ
D. ���������跴Ӧ���ʲ�������Ϊ��Һ�ⵥ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2-����� �ڱ�Ȳ ������ϩ �������� �ݼױ�5���л�������У�����ԭ�Ӳ����ܴ���ͬһƽ���ڵ���( )
A. �٢ڢۢ� B. �٢ڢ� C. �ۢܢ� D. �ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��A��B��C�ֱ��������л���Ľṹģ�ͣ�

��ش��������⣺
��1��A��B����ģ�ͷֱ����л����________ģ�ͺ�________ģ�͡�
��2��A����ͬϵ��ķ���ʽ����ͨʽ________(��n��ʾ)����n��________ʱ��������ʼ����ͬ���칹�塣
��3��A��B��C�����л����У�����ԭ�Ӿ��������________(������)���ṹ��ʽΪCH2=CH(CH2)5CH3���л����У�����ͬһƽ���ڵ�̼ԭ�������Ϊ________��
��4���л���C���еĽṹ��������________(����ĸ)��
a����̼̼˫����̼̼��������Ľṹ
b���ж���������ˮ���ܶȱ�ˮС
c������ʹ����KMnO4��Һ����ˮ��ɫ
d��һ������������������������Ӧ
��5���������������л�����ȫȼ������H2O��CO2���������������(��ͬ״����)������________(�����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��DΪԭ���������������ǰ������Ԫ�ء�BA3��ʹʪ��ĺ�ɫʯ����ֽ������A��B��C����ԭ�ӵĵ�����֮�͵���25��DC������D+��3d�ܼ��ϵ���ȫ������
��ش��������⣺
��1����������Ԫ���У���һ������������________����Ԫ�ط���)�� D�Ļ�̬ԭ�ӵĺ�������Ų�ʽΪ__________��
��2����BA3��AC�У��е�ϸߵ���________�����ѧʽ)����ԭ����____________��DA�ľ���������____________________��
��3��BA4C�����к��еĻ�ѧ��Ϊ_____________��
a�����»��� b����� c�����Ӽ� d����λ�� e�����ۼ�
��4��������BC3�����幹��Ϊ_____________��������ԭ�ӵ��ӻ��������Ϊ______________��
��5����B��D�γɵľ���ľ���ͼ��ʾ����֪���ڵ�Bԭ����Dԭ�Ӿ���Ϊacm��

�ٸþ�����ѧʽΪ___________��
��BԪ��ԭ�ӵ���λ��Ϊ____________��
�۸þ�����ܶ�Ϊ____________���ú�a��NA�Ĵ���ʽ��ʾ����NAΪ�����ӵ�����ֵ��g��cm-3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com