
| A��̼�������Һ����������������Һ��Ӧ + OH��NH3��H2O |
| B��AlCl3��Һ�м��������İ�ˮ��Al3++ 4NH3?H2O�T�TAlO2��+4NH4++2H2O |
| C����������Һ����μ���Ba(OH)2��Һ��SO42-ǡ�ó�����ȫ 2Al3��+ 3SO42-+ 3Ba2��+ 6OH��2Al(OH)3��+ 3BaSO4�� |
| D��FeCl2��Һ��Cl2��Ӧ��2Fe2++Cl2=2Fe3++2Cl�� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������������Һ��Ӧ�� 2Al+ 2OH����2AlO2��+H2�� |
| B��̼��ƺʹ��ᷴӦ��2H+ +Ca CO3�� Ca2++CO2�� + H2O |
| C��ͭ��ϡ���ᷴӦ��Cu + 2H���� Cu2++ H2�� |
| D��SO2ͨ����ˮ�У�SO2+Br2+2H2O=4H++SO42��+2Br�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʯ��ˮ��ͨ�����CO2�� CO2+ O H ��=HCO3 �� |
| B��������Ͷ��ˮ�У�Na + H2O��Na+ + OH��+ H2�� |
| C��������H2SO4��Һ�μӵ�Ba��OH��2��Һ��H++SO42-+OH��+Ba2+=BaSO4��+H2O |
| D�������ۼ��뵽AgNO3��Һ��Fe+Ag+=Ag+Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��CH3COO�� + H2O CH3COOH + OH�� CH3COOH + OH�� | B��NH4+ + H2O NH4OH + H+ NH4OH + H+ |
C��S2- + 2H2O H2S+2OH- H2S+2OH- | D��HCO3- + H2O  H3O++CO32- H3O++CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ˮ�������õķ�������ƿ�м���ϡ���Ὣ���ܽ⣬�䷴Ӧ�����ӷ���ʽΪ��
ˮ�������õķ�������ƿ�м���ϡ���Ὣ���ܽ⣬�䷴Ӧ�����ӷ���ʽΪ�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ���ӷ���ʽ��ȷ����
���ӷ���ʽ��ȷ���� A�����Ƶ�ˮ�ⷴӦ��S2����H3O��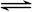 HS����H2O HS����H2O |
| B�����Ȼ�������Һ�м���ϡ���3Fe2+ + 4H+ + NO3-��3Fe3+ + NO�� + 2H2O |
| C����Ca(HCO3)2��Һ�м�������������������Һ�� Ca2��+HCO3��+OH����CaCO3�� + H2O |
D����ͭΪ�缫���ϡ������Һ�� 2H2O  O2�� + 2H2 �� O2�� + 2H2 �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.1 mol/L��Na2CO3��Һ������Ũ�ȹ�ϵΪ c(Na+) = c(HCO3��) + 2c(CO32��) + c(H2CO3) |
| B��1 L 1 mol��L��1��CH3COOH��Һ�У�CH3COO����ĿΪNA |
| C����������ˮ��Ӧ�����ӷ���ʽΪ Na + H2O |
| D����̼��������Һ�м��������������ƣ��䷴Ӧ�����ӷ���ʽΪ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����̼�ᱵ�еμ�ϡ���CO32����2H����CO2����H2O |
| B����С�մ�����θ����ࣺHCO3����H����CO2����H2O |
| C������ˮ�еμ��Ȼ�����Al3����4OH����AlO2����2H2O |
| D������������Һ��ϡ���ᷴӦ��Ba2����SO42����H����OH����BaSO4����H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com