
һ��Ũ��NaHCO3��Һ��CuSO4��Һ��Ӧ������������ɫ����״�������ͳ����ɷ�������������ּ��裺
����һ��������CuCO3���������������Cu(OH)2��
��������������CuCO3��Cu(OH)2�Ļ���
(1)д���������������Cu(OH)2���ɵ����� (�����ӷ���ʽ��ʾ)��
(2)Ϊ��̽�������ijɷ֣�ȡ����һ���ֳ������μ�ϡ���ᣬ������ų���ƾ�������жϳ����к��� ��
(3)Ϊ�˽�һ��̽�������ijɷ֣�����ȷ�������к��ּ�����������ʵ�飬װ��ͼ���£�
���о����������ǰ���뽫��������Һ�з��벢�����������������Ϊ ��ϴ�ӡ����
��װ��E��ҩƷ�������� ����Ϊ ��
��ʵ������������²������裺a.��K1��K3���ر�K2��K4��ͨ������������˲���������� ��
b���ر�K1��K3����K2��K4����ַ�Ӧ��c.��ͨ���������ʱ���������ڴ��� ���رյ��� ��
����������Ʒ������Ϊm g��װ��D������������n g����������ƷΪ�����m�� n֮��Ĺ�ϵΪ ��
����������������Cu(OH)2����������Ϊ ���������в���c�����ʹ��ý�� (�ƫ�ߡ�����Ӱ�족��ƫ�͡�)��
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
ʵ��I����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38��ʱ����Ʒ����NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣
��1��װ�â۵�������
װ�âٵ�������
��2��װ�â��в���ClO2�Ļ�ԭ����
װ�â����Ʊ�ClO2�Ļ�ѧ����ʽΪ
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ��� ���ܵ���60�����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������ƷС���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2-+4I-+4H+=2H2O+2I2+Cl-)�������û��Һ���250mL������Һ��
����ȡ25��00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol��L-1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL(��֪��I2+2S2O32-=2I-+S4O62-)��
��4���ﵽ�ζ��յ�ʱ������Ϊ
��5������Ʒ��NaClO2����������Ϊ (�ú�m��c��V�Ĵ���ʽ��ʾ)��
��6���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�ijУһ�о���ѧϰС��Ե����������ȷֽ�������ۡ�
�����Dz������ϵ�֪���������������ں����м���ʱ����79��134��,����ʧ��14��4����134��250�棬��ʧ��14��4����250��300�棬��ʧ��7��2����֮�������620�棬����ά�ֲ��䡣С�龭������ó���������300��620��Ĺ��������Ϊ��ˮ����ͭ��134��ʱ�Ĺ��������Ļ�ѧʽΪ ��
��С�����ˮ����ͭ�������¼��ȵĿ��ܱ仯���в��롣��������˲�������¼��ֲ��룺
�٣�CuO��SO3����
�ڣ�CuO��SO2��O2��
�ۣ�CuO��SO3��SO2��
�ܣ�CuO��SO3��SO2��O2
С�龭���������ۣ���Ϊ����۲���ʵ��Ϳ��ų������ǵ�������
�������ϣ���SO3Ϊ��ɫ���壬�۵�16��6�棬�е�44��8�档
��SO2���۵�:��72��4�棬�е�:��10�棻SO2ͨ��BaCl2��Һ�У�����������
��ʵ��̽����
С�鰴��ͼ��ʾ��װ��ʵ��װ�á�
��1����װ��װ�ú�δװҩƷǰ������еIJ����� ��
Dװ�õ������� ��
��2����ͼʾװ��ҩƷ���þƾ���ƶ���Ӳ���Թܼ��ȡ�һ�����B����Һ������ɫ���ǣ�C����Һ����ɫ��
����ʵ����������
��1��С��ͬѧ�����Ϊ��ˮ����ͭ�ȷֽ����Ӧ��Ϊ����ܡ�����һ��ͬѧ������ɣ�����ΪB����Һ������ɫ���Dz���һ����ȷ�������к���SO3�����������漰�Ļ�ѧ����ʽ�� �����ǣ�С��ͬѧ�����۾�����������һ��װ��E������Ϊ��װ��Ӧ���� ����װ����ĸ��֮�䡣����װ�ú�С������ʵ�飬֤���˲�����ȷʵ����SO3������Ϊ���Ǹ���ʲô����õ���һ���ۣ� ��
��2��С���������ˮ����ͭ�ȷֽ�Ļ�ѧ����ʽʱ���������ѡ����Ƿ��ָû�ѧ����ʽΪ��������ʽ��������������ƽ������������ܵط�����������ΪֻҪ��ȷ��ijЩ���ʵļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������֪SO2��SO3�ļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������SO2��SO3�ļ�����֮��Ϊx����д����ƽ��Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������㷺Ӧ������ҩ�ͻ�����ҵ��ij��ȤС��ͬѧ���ø�����������ױ��Ʊ������ᣨKMnO4���������»�ԭ����ΪMnO2������������ΪMn2+��
��֪����������Է�����122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3 g��6.9 g��
ʵ�����̣�
����װ��A�м���2.7mL��2.3 g���ױ���125 mLˮ��Ȼ������μ���8.5 g�Թ�����KMnO4���壬���Ʒ�Ӧ�¶�Լ��100�棬���ױ��������ʧ���������ٳ�������ʱ��ֹͣ���ȡ�
�ڽ���Ӧ�������ȹ��ˣ���������ˮϴ���������ϲ���Һ��ϴ��Һ����ȴ�����Ũ���ᣬ������I�ð�ɫ�ϸ���ֲ�Ʒ��
�۴��Ȳⶨ����ȡ1.220g��ɫ��Ʒ�����100mL,�״���Һ��ȡ25.00mL��Һ����0.1000mol/L KOH����Һ�ζ����ظ��ζ��ĴΣ�ÿ�����ĵ�������±���ʾ�о٣�
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����mL) | 24.00 | 24.10 | 22.40 | 23.90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
TiO2�����Ʊ��������ѻ������ԭ�ϣ�����һ����������İ�ɫ���ϡ�
��1��ʵ�������÷�ӦTiO2��s��+2CCl4��g����TiCl4��g��+CO2��g��������ˮ���������£���ȡTiCl4ʵ��װ��ʾ��ͼ���£�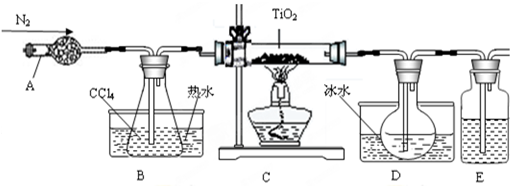
�й��������±�
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ������������ |
 H2TiO3��s��+H2SO4��aq��
H2TiO3��s��+H2SO4��aq��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������2-�ǻ����������������ڵ��ƹ����͡�������ʳ�ú;����㾫��Ϊ����ʵ������ȡ����������ij�о���ѧϰС��ͬѧ���Ȳ������ϣ����������Ϣ��
�ٲ������ʵķе㣺
| �� �� | ˮ | �Ҵ� | ���� | �� | �������� |
| �е㣯�� | 100 | 78.4 | 122 | 80.10 | 154 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ijС��ͬѧ��һ��Ũ��NaHCO3��Һ���뵽CuSO4��Һ�з��������˳�������ͬѧ��Ϊ������CuCO3����ͬѧ��Ϊ������CuCO3��Cu(OH)2�Ļ����������ʵ��ͨ���ⶨ������CuCO3������������ȷ����������ɡ�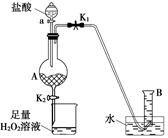
��1�����ռ�ͬѧ�Ĺ۵㣬������Ӧ�����ӷ���ʽΪ�� ��
��2����ͬѧ������ͼ��ʾװ�ý��вⶨ��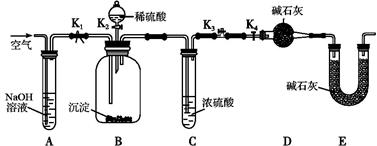
�����о����������ǰ���뽫��������Һ�з��벢�����������������Ϊ_____��_____�����
��װ��E�м�ʯ�ҵ������� ��
��ʵ������������²�������:
a���ر�K2��K4����K1��K3��ͨ���������
b���ر�K1��K3����K2��K4����ַ�Ӧ
c���ر�K2��K3����K1��K4��ͨ���������
����������Ϊm��C��Ũ��������x��D�м�ʯ������Ϊy����CuCO3����������Ϊ______����δ���в���a����ʹ������� ��
��ijͬѧ�����ͼ��ʾװ�ã��г�װ���ԣ��������Ѽ��飩���÷���м����ȡFeCl3��6H2O���塣������������£�
�ٴ��ɼ�K1���رյ��ɼ�K2��������a�������μ�������������
�ڵ�����ʱ���رյ��ɼ�K1�����ɼ�K2����A����Һ��ȫ�����ձ���رջ���a��
�۽��ձ��к����������Һ����Ũ������ȴ�ᾧ�����˺�õ�FeCl3��6H2O���塣
��ش�
��1�����������������������_____��
��2��д��A����Һ�����ձ�������Ӧ�����ӷ���ʽ��____��
��3��������ձ�����Һ�������������____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
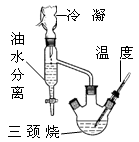
2-����-2-��������Ҫ���л��ϳ��м��壬���屽Ϊԭ�Ϻϳɸ��м���ķ�Ӧԭ�����£�
��ʵ��װ�á�

װ�â� װ�â�
��ʵ�鲽�衿
����1����þ������װ�â��������ƿ�У�����100 mL���ѣ���=0.71g��cm��3��������ȴ�����»��������屽���Ȳ�����һС���������Ӧ�١�
����2���μ�14.2 mL��ͪ��30 mL���ѻ���������Ӧ�ڣ��μ���20����NH4Clˮ��Һ��������Ӧ�ۡ�
����3���ֳ��Ͳ㣬��ˮϴ�������ԣ�����ˮCaCl2���
����4����װ�â����������ѣ����������Ʒ��
�Ų���1�м����������� ��
��װ�â��еμ�Һ�����õ������������� ����Ӧ���轫������ƿ���ڱ�ˮ�У�����μ��붡ͪ�����ѣ���Ŀ���� ��
�Dz������з�����Ͳ�ľ���ʵ������� ��
��װ�â���õ��Ǽ�ѹ����ʵ��ʱ�轫����ƿ������ ��������������ʽ��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���Һϳɻ���ͪ�ķ�Ӧ��װ��ʾ��ͼ���й��������£�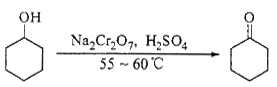

������������ͪ������ʳ��ˮ��ˮ�IJ����������ʼ��±���
| ���� | �е�(��) | �ܶ�(g��cm��3, 20��) | �ܽ��� |
| ������ | 161.1(97.8) | 0.9624 | ������ˮ |
| ����ͪ | 155.6(95) | 0.9478 | ����ˮ |
| ����ʳ��ˮ | 108.0 | 1.3301 | |
| ˮ | 100.0 | 0.9982 | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com