
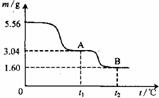
 ��ͼ��5.56g�̷�(FeSO4��7H2O)�����������ȷֽ�ʱ�����ù��������������¶ȱ仯�����ߡ������Ȳ�������������ͨ��������BaCl2��Һ���ð�ɫ����2.33g������˵������ȷ����
��ͼ��5.56g�̷�(FeSO4��7H2O)�����������ȷֽ�ʱ�����ù��������������¶ȱ仯�����ߡ������Ȳ�������������ͨ��������BaCl2��Һ���ð�ɫ����2.33g������˵������ȷ����
A���������Ը��������Һ����FeSO4��Һ�Ƿ����
B���¶�Ϊt2ʱ������B�Ļ�ѧʽΪFeO
C���ڸ�������������A�õ�B�Ļ�ѧ����ʽΪ
FeSO4![]() FeO+SO3
FeO+SO3
D������2.88g��������(FeC2O4)��������������100�棬�õ�1.44g��ɫϸ�ۣ������֪�ú�ɫϸ��ΪFeO
D
��FeSO4��Һ���ʣ�����Һ�к���Fe3+������������Һ���Ƿ���Fe3+���ܴﵽʵ��Ŀ�ģ���Ȼʹ�����Ը��������Һ�Ǵ���ģ���ѡKSCN��Һ��5.56g�̷��൱��0.02mol������0.14mol�ᾧˮ��2.52g�����¶�Ϊt1ʱ�����干ʧ��5.56-3.04=2.52g������ȫ���ǽᾧˮ���������ɴ˿�֪�¶�Ϊt1ʱ�̷���ȫʧˮ�õ�FeSO4���������⽫���Ȳ�������������ͨ��������BaCl2��Һ���ð�ɫ����2.33g���ð�ɫ����ӦΪBaSO4��������0.01molSO42-��С���̷�������SO42-�����ʵ���������ڼ��ȹ����б���SO2�������ɣ�B����������1.60g�����ȹ�������Ԫ�ز�����ʧ��������B����Ԫ�ص�����Ϊ��1.60-0.02��56=0.48g���൱��0.03mol����˿ɵù���B�Ļ�ѧʽΪFe2O3������A��B������ѧ��Ӧ�ķ���ʽӦΪ2FeSO4![]() Fe2O3+SO2��+SO3����ͬ��������Ԫ���غ�ɵø������²��������ȷֽ�Ļ�ѧ����ʽΪ��FeC2O4
Fe2O3+SO2��+SO3����ͬ��������Ԫ���غ�ɵø������²��������ȷֽ�Ļ�ѧ����ʽΪ��FeC2O4![]() FeO+CO��+CO2�������Ա���ӦѡAD��
FeO+CO��+CO2�������Ա���ӦѡAD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com