
| ��� | ʵ����� | ʵ����������� |
| �� | ȡ������ĩ�����Թ��У�ע��1mol/L������ | ��ĩ���ܽ⣬��Һ�ʻ���ɫ |
| �� | �����١���������Һ�ֳ����ݣ�������һ�ݵμӼ���KSCN��Һ���� | ����Һ��Ϊ Ѫ��ɫ Ѫ��ɫ ��˵����Fe2O3���� |
| �� | ����һ���м�������KMnO4��Һ | ����Һ ��ɫ��ȥ ��ɫ��ȥ ��˵����FeO���� |
| Ũ���� |
| �� |
| a+2b |
| a+3b |
| 4 |
| 5 |
| a+2b |
| a+3b |
| 4 |
| 5 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
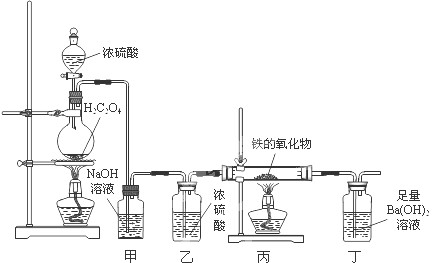
ij�����������ĩ���ܺ���FeO��Fe2O3�е�һ�ֻ����֡�ij��ȤС��ͬѧ��������װ�òⶨ�������������ɣ���ش��������⣺
����֪ ��
��
��1��װ�ü������� ��
��2��װ�ö��е�ʵ�������� ��
��3������������������Ļ�ѧʽΪFexOy������з�Ӧ�Ļ�ѧ����ʽΪ ����������������������Ϊ3.92g������ȫ��Ӧ���������ɳ���������Ϊ13.79g����ȷ������������x : y= ���ù����ĩ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ������ѧ�߶���ѧ����ĩ���Ի�ѧ���⣨���ޣ� ���ͣ������
ij�����������ĩ���ܺ���FeO��Fe2O3�е�һ�ֻ����֡�ij��ȤС��ͬѧ��������װ�òⶨ�������������ɣ���ش��������⣺
����֪ ��
��
��1��װ�ü������� ��
��2��װ�ö��е�ʵ�������� ��
��3���������� ��������Ļ�ѧʽΪFexOy������з�Ӧ�Ļ�ѧ����ʽΪ ����������������������Ϊ3.92g������ȫ��Ӧ���������ɳ���������Ϊ13.79g����ȷ������������x : y= ���ù����ĩ�����Ϊ ��
��������Ļ�ѧʽΪFexOy������з�Ӧ�Ļ�ѧ����ʽΪ ����������������������Ϊ3.92g������ȫ��Ӧ���������ɳ���������Ϊ13.79g����ȷ������������x : y= ���ù����ĩ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�߶���ѧ����ĩ���Ի�ѧ���⣨���ޣ� ���ͣ������
ij�����������ĩ���ܺ���FeO��Fe2O3�е�һ�ֻ����֡�ij��ȤС��ͬѧ��������װ�òⶨ�������������ɣ���ش��������⣺
����֪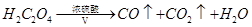 ��
��
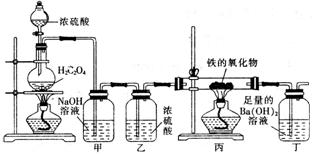
��1��װ�ü������� ��
��2��װ�ö��е�ʵ�������� ��
��3������������������Ļ�ѧʽΪFexOy������з�Ӧ�Ļ�ѧ����ʽΪ ����������������������Ϊ3.92g������ȫ��Ӧ���������ɳ���������Ϊ13.79g����ȷ������������x : y= ���ù����ĩ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ʵ����� | ʵ����������� |
| �� | ȡ������ĩ�����Թ��У�ע��1mol/L������ | ��ĩ���ܽ⣬��Һ�ʻ���ɫ |
| �� | �����١���������Һ�ֳ����ݣ�������һ�ݵμӼ���KSCN��Һ���� | ����Һ��Ϊ______��˵����Fe2O3���� |
| �� | ����һ���м�������KMnO4��Һ | ����Һ______��˵����FeO���� |
 CO��+CO2��+H2O������������װ�ý��ж���̽����
CO��+CO2��+H2O������������װ�ý��ж���̽����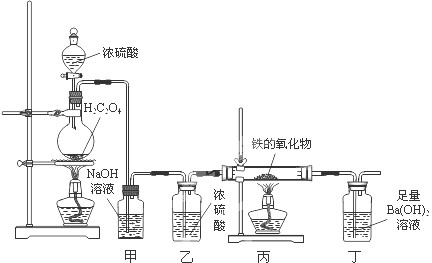
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com