
| ���� | ��ʼ����ʱ��PH | ��ȫ����ʱ��PH |
| Fe3+ | 1.9 | 3.2 |
| Zn2+ | 6.4 | 8.0 |


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
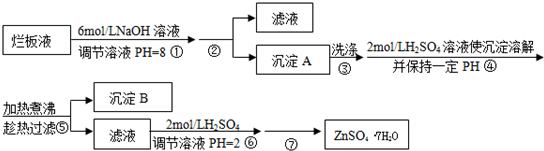
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£�

��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
(1)���ð�Һ�������ʵ���Ҫ�ɷ���________(�ѧʽ)����ϡ���ḯʴп�����������ΪN2O��д��ϡ���ḯʴп�巴Ӧ����Ҫ��ѧ����ʽ_____________________________��
(2)�ڲ������б���pH��8��Ŀ����____________________________��
(3)���������Ҫ�ɷ���_______________________________________��
(4)�������м��ȡ���е�Ŀ����________________________________��
�˲������������������____________________________________��
(5)�����ܱ���pH��2��Ŀ����__________________________________��
�˲�����������õ���Ҫ������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£�
��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
(1)���ð�Һ�������ʵ���Ҫ�ɷ���________(�ѧʽ)����ϡ���ḯʴп�����������ΪN2O��д��ϡ���ḯʴп�巴Ӧ����Ҫ��ѧ����ʽ_____________________________��
(2)�ڲ������б���pH��8��Ŀ����____________________________��
(3)���������Ҫ�ɷ���_______________________________________��
(4)�������м��ȡ���е�Ŀ����________________________________��
�˲������������������____________________________________��
(5)�����ܱ���pH��2��Ŀ����__________________________________��
�˲�����������õ���Ҫ������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ����ʮһ�и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£�
��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
(1)���ð�Һ�������ʵ���Ҫ�ɷ���________(�ѧʽ)����ϡ���ḯʴп�����������ΪN2O��д��ϡ���ḯʴп�巴Ӧ����Ҫ��ѧ����ʽ_____________________________��
(2)�ڲ������б���pH��8��Ŀ����____________________________��
(3)���������Ҫ�ɷ���_______________________________________��
(4)�������м��ȡ���е�Ŀ����________________________________��
�˲������������������____________________________________��
(5)�����ܱ���pH��2��Ŀ����__________________________________��
�˲�����������õ���Ҫ������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£�

��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
(1)���ð�Һ�������ʵ���Ҫ�ɷ���________(�ѧʽ)����ϡ���ḯʴп�����������ΪN2O��д��ϡ���ḯʴп�巴Ӧ����Ҫ��ѧ����ʽ_____________________________��
(2)�ڲ������б���pH��8��Ŀ����____________________________��
(3)���������Ҫ�ɷ���_______________________________________��
(4)�������м��ȡ���е�Ŀ����________________________________��
�˲������������������____________________________________��
(5)�����ܱ���pH��2��Ŀ����__________________________________��
�˲�����������õ���Ҫ������________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com