
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ֪�������·�Ӧ��
֪�������·�Ӧ��
 �����ּ���F�еĵͼ۽��������ӵ�ʵ�鷽��������ʵ�鲽�輰Ԥ��ʵ������
�����ּ���F�еĵͼ۽��������ӵ�ʵ�鷽��������ʵ�鲽�輰Ԥ��ʵ������ ��Һ����Һ����ɫ���ٵμ���ˮ����Һ���ɫ��
��Һ����Һ����ɫ���ٵμ���ˮ����Һ���ɫ�� NaOH��Һ�����ɰ�ɫ������Ȼ��Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
NaOH��Һ�����ɰ�ɫ������Ȼ��Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢۢ� | B���ڢۢܢ� | C���٢ۢ� | D���٢ڢۢ� |
| ��� | �� | �� | �� |
| �� | CO2 | SO2 | ʯ��ˮ |
| �� | HCl | CO2 | ʯ��ˮ |
| �� | CO2 | SO2 | B a(NO3)2 a(NO3)2 |
| �� | NO2 | SO2 | BaCl2 |
| �� | CO2 | NH3 | CaCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
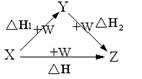
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������������뵼����� | B�����᳣��������ͭ�ӹ�ǰ����ϴ |
| C���������ƿ���������θ����� | D��������������������ɫ�����Ϳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 aHCO3 + HCl���ڲ�ͬ�������¶ȡ�Ũ�ȡ������ȣ����ܵõ���ͬ�������
aHCO3 + HCl���ڲ�ͬ�������¶ȡ�Ũ�ȡ������ȣ����ܵõ���ͬ�������| A���٢ڢۢܢޢ� | B���٢ڢۢܢ� | C���٢ڢۢܢ� | D���٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com