
(15��)���������Ǵ�����Ⱦ��֮һ��Ϊ���Բⶨ��Χ�����ж�������ĺ�����ij����С��ļס�����λͬѧ�ֱ�����ͬʵ��װ�ú���Һ���ⶨͬһʱ�䣬 ͬһ�ص����(��SO2��N2��O2���壬�����������)��SO2�ĺ�����ʵ��װ������ͼ��Ӧ�Թ���װ�е�ĵ���ϡ��ҺA�� SO2��I2������ӦΪ��S02+I2+2H20=H2SO4+2HI(N2��02����I2�� ���۷�Ӧ)���Իش��������⣺
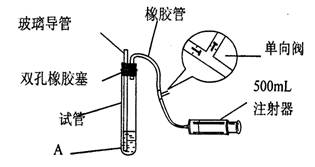
(1)����װ��������ʱ�������Թ���װ��������ˮ(��֤�����ܵ��¶˽���ˮ��)��Ȼ��_____________________(ע�ⷧ�ĵ�����)����֤����װ�õ����������á�
(2)��A��Һ�����ΪVmL��Ũ��Ϊc mol��L-1������Һ����ɫ�պñ�ɫ��ֹͣ��������ʱ�ס�����λͬѧע�����ڳ�����������ֱ�ΪV��mL��V��mL(���е����������ɱ�������)����V��>V�ң���ס��������ⶨ�������õص������S02�����������ʵ�����ӽ�����_______________________(.�ú�c��V��V����V�����ȵĹ�ϵʽ��ʾ)����һλʵ���������ϴ�����ԭ�������_______________________________________________
(3)��������װ�ý��иĽ����������⣬��������װ����Ҫѡ�õ�������___________________�� (ѡ���������ı��)
a���ձ� b���Թ� c����ƿ d������ƿe����Ͳ f�������� g.˫����
(4)��������������ȥ�����еĶ��������ѡ�õ��Լ���___________________��
(1) ������������ע�����������Թ��ڵ��ܿ�������ð��
(2) ���������죬SO2 δ����ˮ�������
���������죬SO2 δ����ˮ�������
(3) beg��ceg��bceg (ֻҪ�𰸺���������)
(4) ����Һ�� (�й������������������𰸾��ɸ���)
����������1�����װ���Dz�©���ģ���������������ע�����������Թ��ڵ��ܿ�������ð�����ݴ˿��Լ��顣
��2����ͬѧ�ó����ʻ�ƫ����˵���������죬SO2 û����ȫ����ˮ������գ����Ӧ������ͬѧ�Ľ��м��㡣���ݷ���ʽ��֪�����ĵĵ��ʵ���0.001Vcmol��ʵ��SO2�������0.0224VcL�����SO2�ĺ����� ��
��
��3��������Ҫ������������Ա�������Ͳ����˴��� beg��ceg��bceg��
��4��SO2����������������ü�Һ���ա�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)
���Ṥ���ŷŵ�β���У��������Ķ�������Ϊ��ֹ��Ⱦ���������ŷ�ǰ�������β���������跨�����ۺ����á�
�����Ṥ���ŷ�β���е�SO2ͨ��������ʯ��ˮ���գ�Ȼ������ϡ���ᴦ����
��д���������̵Ļ�ѧ��Ӧ����ʽ�� ��
����˵������β�������������ŵ㣨˵�����㼴�ɣ�
����ij���᳧ÿ���ŷŵ�1����3������״����β���к�0.2%������ٷ�������SO2��ͨ��������������������������ʯ�� kg��
�ƽ����������ձ������о���Na2SO3���շ���Ϊ����SO2��Ⱦ��һ���·������÷�������һ������Na2SO3ˮ��Һ����SO2���ڶ����Ǽ���������Һ���ɵõ�����Ũ��SO2��ˮ��������Ʒ��
����β�����������루1����ȵ��ŵ��� ��
��ij�о�С����NaOH��Һ����β���еĶ����������õ�Na2SO3��Һ���е��ѭ�������������¹��ս�����ѭ��������������Ĥ���ѭ������������ͼ��a��b���ӽ���Ĥ�����۷�Ϊ���������缫����Ϊʯī��
��ͼ��a��ʾ ���ӽ���Ĥ�����������������A��E�ֱ���������е�ԭ�ϻ��Ʒ������CΪ���ᣬ��A��ʾ ��E��ʾ ��
�������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)������ͼ��ʾװ�ü�����ҩƷ̽����ҵ������ʱ���������ת���ʡ���֪C�к�����n mol�����������C��Dʱ�ֱܷ���ȫ����ijһ�����壬�Һ���װ���ڿ����е�CO2��
��ش��������⣺
��1�������װ�õ��������Ҽ���ҩƷ��ʼ����ʱ����Ӧ���еIJ�����
��
��2��ͨ���۲������ݵ��ݳ����ʿɿ���SO2��O2������������ȣ�Aװ�û���������� ��
��3��Ϊ����߲ⶨSO2ת���ʵ�ȷ�ȣ����в�����Ľ����е��ǣ�����ţ� ��
��Ϩ��ƾ��ƺ�ֹͣͨ��SO2������ͨ��O2һ��ʱ��
��Ϩ��ƾ��ƺ�ֹͣͨ��O2������ͨ��SO2һ��ʱ��
����C��D֮���װһ��ʢ��Ũ�����ϴ��ƿ
��4�������徭����֣�ʵ�������ϡ��װ��C�е���Һ���������м���������BaCl2��Һ���õ��ij�������Ϊw g����װ��D���ӵ�����Ϊa g������������ת�����ǣ�
���ú���ĸ�Ĵ���ʽ��ʾ������ɲ�����
��5������Ӵ�����ͨ��2 molSO2(g)��1molO2(g)����һ���¶��£���Ӧ�ﵽƽ��ʱ��ýӴ����ڵ�ѹǿΪ��ʼʱ��0.75������SO2��ת����Ϊ ����ͬ�����£�����ʼʱͬʱͨ��a mol SO2��bmol O2��c mol SO3(g)ʱ���ﵽƽ��ʱ��ԭƽ���Ч������ʼʱά�ֻ�ѧ��Ӧ���淴Ӧ������У���c��ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��������и����ڶ����¿������Ծ�����ѧ���֣� ���ͣ������
(15��)�ش��������⡣
(1) Ư����Ũ��������ʱ�л���ɫ�������ɣ������������ڱ�״���µ����Ϊ11.2L������ molHCl��������
(2) ����ˮ�м���CaCO3�������ˮ��ɱ����Ư�������� ������ǿ���������ԭ���� ��
��3���������������뺬1.5 mol ClԪ�ص�һ�ֺ����ᣨ�����ij�γ�����ʵ������ȡ����������Һ��һ�������·�Ӧ��������һ��ǿ���һ����̬��������A������1.5��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ����������� ������ ��
��4���ҹ�����������ȷ�����Ҫ�����ȵ�������A���������������ˮ��������
���ȵ�������A���ȶ�������NaOH��Һ��H2O2��Ӧ��ClԪ��ת��Ϊ�Ƚ��ȶ������������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
�� ���ȵ�������A������������ˮ�л��������A������FeCl2���仹ԭΪ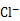 ������VL(�ѻ���Ϊ��״��) A����һ��������ˮ���ټ���a mol FeCl2�ɽ�������Aǡ�ó�ȥ���������ˮ�в���A�����ʵ���Ϊ ��
������VL(�ѻ���Ϊ��״��) A����һ��������ˮ���ټ���a mol FeCl2�ɽ�������Aǡ�ó�ȥ���������ˮ�в���A�����ʵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����ж��и�����ѧ���ܲ⻯ѧ�Ծ����������� ���ͣ�ʵ����
(15��)���������Ǵ�����Ⱦ��֮һ��Ϊ���Բⶨ��Χ�����ж�������ĺ�����ij����С��ļס�����λͬѧ�ֱ�����ͬʵ��װ�ú���Һ���ⶨͬһʱ�䣬 ͬһ�ص����(��SO2��N2��O2���壬�����������)��SO2�ĺ�����ʵ��װ������ͼ��Ӧ�Թ���װ�е�ĵ���ϡ��ҺA�� SO2��I2������ӦΪ��S02+I2+2H20=H2SO4+2HI(N2��02����I2�� ���۷�Ӧ)���Իش��������⣺
(1)����װ��������ʱ�������Թ���װ��������ˮ(��֤�����ܵ��¶˽���ˮ��)��Ȼ��_____________________(ע�ⷧ�ĵ�����)����֤����װ�õ����������á�
(2)��A��Һ�����ΪVmL��Ũ��Ϊc mol��L-1������Һ����ɫ�պñ�ɫ��ֹͣ��������ʱ�ס�����λͬѧע�����ڳ�����������ֱ�ΪV��mL��V��mL(���е����������ɱ�������)����V��>V�ң���ס��������ⶨ�������õص������S02�����������ʵ�����ӽ�����_______________________(.�ú�c��V��V����V�����ȵĹ�ϵʽ��ʾ)����һλʵ���������ϴ�����ԭ�������_______________________________________________
(3)��������װ�ý��иĽ����������⣬��������װ����Ҫѡ�õ�������___________________�� (ѡ���������ı��)
a���ձ� b���Թ� c����ƿ d������ƿe����Ͳ f�������� g.˫����
(4)��������������ȥ�����еĶ��������ѡ�õ��Լ���___________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com