

 ���ʴ�Ϊ��K=
���ʴ�Ϊ��K= ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| c2(CO) |
| c(CO2) |
| c2(CO) |
| c(CO2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
����ת¯ú��[CO��60~80%����CO2��15~20%������N2��]�����Ṥҵβ���е�SO2�����ܾ���β�������ܻ�ñ��շۣ�Na2S2O4�����䲿�ֹ����������£�
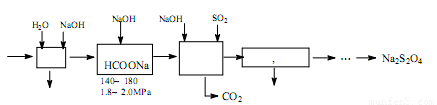
��1��ת¯����ʱ�����ڷ�Ӧ�� ����ƽ�ⳣ������ʽΪK= ��
����ƽ�ⳣ������ʽΪK= ��
��2��ú������ʱ������ˮϴ����NaOH��Һϴ�ӣ���Ŀ���� ��
��3������Һ�л��ռ״��IJ��������� �����ɻ��յ����������� ��ֻдһ�ֻ�ѧʽ����
��4���ϳɱ��շ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��5�����շۡ�H2O2��������ֽ��Ư����д�����շ��������H2O2��ˮ��Һ�з�Ӧ���������ε����ʵ����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�Ͼ��С��γ��и����꼶��һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
����ת¯ú��[CO��60~80%����CO2��15~20%������N2��]�����Ṥҵβ���е�SO2�����ܾ���β�������ܻ�ñ��շۣ�Na2S2O4�����䲿�ֹ����������£�

��1��ת¯����ʱ�����ڷ�Ӧ��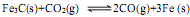 ����ƽ�ⳣ������ʽΪK= ��
����ƽ�ⳣ������ʽΪK= ��
��2��ú������ʱ������ˮϴ����NaOH��Һϴ�ӣ���Ŀ���� ��
��3������Һ�л��ռ״��IJ��������� �����ɻ��յ����������� ��ֻдһ�ֻ�ѧʽ����
��4���ϳɱ��շ۷�Ӧ�Ļ�ѧ����ʽΪ ��
��5�����շۡ�H2O2��������ֽ��Ư����д�����շ��������H2O2��ˮ��Һ�з�Ӧ���������ε����ʵ����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com