
 Ԫ�����ڱ��е������ڵĽ���Ԫ���������Ϳ������зdz���Ҫ��ʹ�ü�ֵ��
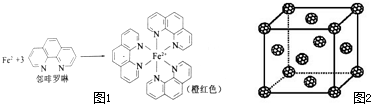
Ԫ�����ڱ��е������ڵĽ���Ԫ���������Ϳ������зdz���Ҫ��ʹ�ü�ֵ�� �����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��kԭ�ӣ�1+6��
�����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��kԭ�ӣ�1+6��| 1 |
| 2 |
 �����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��Kԭ�ӣ�1+6��
�����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��Kԭ�ӣ�1+6��| 1 |
| 2 |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��һ�绯ѧʵ��װ�ã�ͼ��C��D��Ϊ���缫��UΪ���ţ�G�����������ƣ���ָ������ƫ���Դ������
��ͼ��һ�绯ѧʵ��װ�ã�ͼ��C��D��Ϊ���缫��UΪ���ţ�G�����������ƣ���ָ������ƫ���Դ������| [H+]2?[I-]2?[AsO43-] |
| [I2]?[AsO33-] |
| [H+]2?[I-]2?[AsO43-] |
| [I2]?[AsO33-] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com