
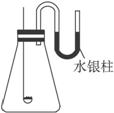
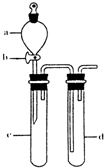
(1)ˮ���������ҹ�˵��___________________________________________________��
(2)���δȼ��ʱ�����Ϩ���ˣ�˵��__________________________________________��
(3)ˮ��������ֻص�ԭ�ȱ궨�Ŀ̶ȣ����ܵó��Ľ�����_____________________________��
 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��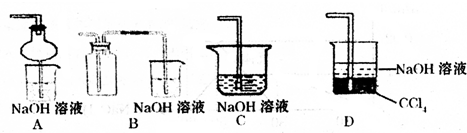
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
��1�������й������˵����ȷ���� ������ţ���
A. SO2��NO2��CO2���ᵼ��������γ�
B. NO������ˮ�����Բ������������Ⱦ
C. ȼúʱ�����������������Լ�������IJ���
D. ���������Դ�����Լ�������IJ���
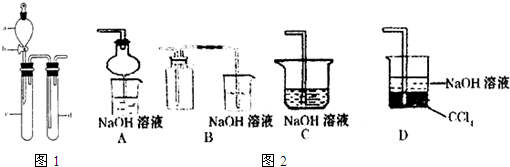
��2��ij��ȤС�������ͼװ����ȡ��̽��SO2��������ʡ�
������ʵ�鷽������������ͼ��ʾװ����ȡ����SO2���� ������ţ���
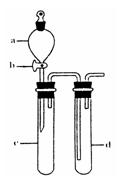
A. Na2SO3��Һ��HNO3 B. Na2SO3������Ũ����
C. �������ڴ�����ȼ�� D.ͭ��ŨH2SO4
����װ���������װ�������Եķ����ǣ��رջ���b��
��ָ����ʦָ��Ӧ����һβ������װ�ã�������ͬѧ�����������װ�ã����к�������
������ţ���
��С��ͬѧ������Թ�d�м���FeCl3��Һ����֤SO2�Ļ�ԭ�ԡ�Ϊ����֤SO2��Fe3+������������ԭ��Ӧ��������ͨ������SO2��ȡ�Թ�d�е���Һ���ֳ����ݣ������������ʵ�飺
����A������һ����Һ�м���KmnO4��Һ���Ϻ�ɫ��ȥ
����B�����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ���
����C������������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ���� ���ͣ�ʵ����
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
��1�������й������˵����ȷ���� ������ţ���
A. SO2��NO2��CO2���ᵼ��������γ�
B. NO������ˮ�����Բ������������Ⱦ
C. ȼúʱ�����������������Լ�������IJ���
D. ���������Դ�����Լ�������IJ���
��2��ij��ȤС�������ͼװ����ȡ��̽��SO2��������ʡ�
������ʵ�鷽������������ͼ��ʾװ����ȡ����SO2���� ������ţ���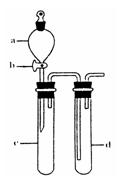
A. Na2SO3��Һ��HNO3 B. Na2SO3������Ũ����
C. �������ڴ�����ȼ�� D.ͭ��ŨH2SO4
����װ���������װ�������Եķ����ǣ��رջ���b��
��ָ����ʦָ��Ӧ����һβ������װ�ã�������ͬѧ�����������װ�ã����к�������
������ţ���
��С��ͬѧ������Թ�d�м���FeCl3��Һ����֤SO2�Ļ�ԭ�ԡ�Ϊ����֤SO2��Fe3+������������ԭ��Ӧ��������ͨ������SO2��ȡ�Թ�d�е���Һ���ֳ����ݣ������������ʵ�飺
����A������һ����Һ�м���KmnO4��Һ���Ϻ�ɫ��ȥ
����B�����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ���
����C������������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ���� ���ͣ�ʵ����
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
��1�������й������˵����ȷ���� ������ţ���
A. SO2��NO2��CO2���ᵼ��������γ�
B. NO������ˮ�����Բ������������Ⱦ
C. ȼúʱ�����������������Լ�������IJ���
D. ���������Դ�����Լ�������IJ���
��2��ij��ȤС�������ͼװ����ȡ��̽��SO2��������ʡ�
������ʵ�鷽������������ͼ��ʾװ����ȡ����SO2���� ������ţ���
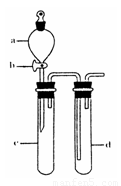
A. Na2SO3��Һ��HNO3 B. Na2SO3������Ũ����
C. �������ڴ�����ȼ�� D.ͭ��ŨH2SO4
����װ���������װ�������Եķ����ǣ��رջ���b��
��ָ����ʦָ��Ӧ����һβ������װ�ã�������ͬѧ�����������װ�ã����к�������
������ţ���
��С��ͬѧ������Թ�d�м���FeCl3��Һ����֤SO2�Ļ�ԭ�ԡ�Ϊ����֤SO2��Fe3+������������ԭ��Ӧ��������ͨ������SO2��ȡ�Թ�d�е���Һ���ֳ����ݣ������������ʵ�飺
����A������һ����Һ�м���KmnO4��Һ���Ϻ�ɫ��ȥ
����B�����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ���
����C������������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ԭ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com